Modeling the Viscosity of Ionic Liquids and Their Mixtures Using ePC-SAFT and Free Volume Theory with an Ion-Based Approach
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
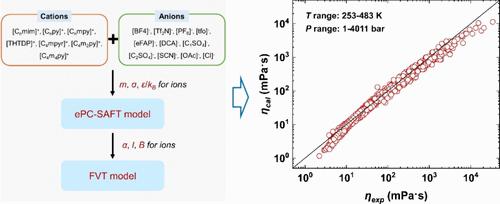
In this work, we developed the electrolyte perturbed-chain statistical associating fluids theory (ePC-SAFT) coupled with free volume theory (FVT) using an ion-based approach (i.e., treating IL cation and anion as distinct species) to model the viscosities of 72 ionic liquids (ILs) across various temperatures and pressures. To evaluate the model performance, we compared the ePC-SAFT-FVT model employing a molecular-based approach (i.e., treating IL as a single pure substance) developed in our previous work. The results indicate that the ion-based approach demonstrates desirable performance, achieving an average ARD of 8.73%. This is comparable to the molecular-based approach, which has an average ARD of 6.09%. Importantly, the ion-based approach requires fewer adjustable parameters, reducing the number from 216 to 81 for 72 ILs, and offers enhanced flexibility by allowing the combination of both cation and anion parameters for predictions. Additionally, the ion-specific ePC-SAFT-FVT model was employed to predict the viscosities of IL mixtures, which were then compared to experimental data of 19 IL mixtures. The findings reveal that the model effectively predicts the viscosity of most IL mixtures, achieving an average ARD of 9.1%. Furthermore, the ion-based approach demonstrates superior predictive performance compared to the molecule-specific ePC-SAFT-FVT model. This study indicates that the ePC-SAFT-FVT model, using an ion-based approach, reliably represents the viscosity of pure ILs and IL mixtures, leveraging the flexibility of cation and anion parameter combinations to enhance predictive capabilities.
离子液体及其混合物的黏度建模:基于离子基方法的ePC-SAFT和自由体积理论
在这项工作中,我们开发了电解质微动链统计关联流体理论(ePC-SAFT)和自由体积理论(FVT),使用基于离子的方法(即,将IL阳离子和阴离子作为不同的物种)来模拟72种离子液体(IL)在不同温度和压力下的粘度。为了评估模型的性能,我们采用基于分子的方法(即将IL视为单一纯物质)比较了我们之前工作中开发的ePC-SAFT-FVT模型。结果表明,离子基方法具有良好的性能,平均ARD为8.73%。这与基于分子的方法相当,后者的平均ARD为6.09%。重要的是,基于离子的方法需要更少的可调参数,将72个il的数量从216个减少到81个,并且通过允许结合正离子和阴离子参数进行预测,提供了更高的灵活性。此外,采用离子特异性ePC-SAFT-FVT模型预测了IL混合物的粘度,并与19种IL混合物的实验数据进行了比较。结果表明,该模型能有效预测大多数IL混合物的粘度,平均ARD达到9.1%。此外,与分子特异性ePC-SAFT-FVT模型相比,基于离子的方法显示出更好的预测性能。该研究表明,ePC-SAFT-FVT模型采用基于离子的方法,可靠地代表了纯IL和IL混合物的粘度,利用正离子和阴离子参数组合的灵活性来增强预测能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


