Design, Synthesis, and Pharmacological Evaluation of Nonsteroidal Tricyclic Ligands as Modulators of GABAA Receptors
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
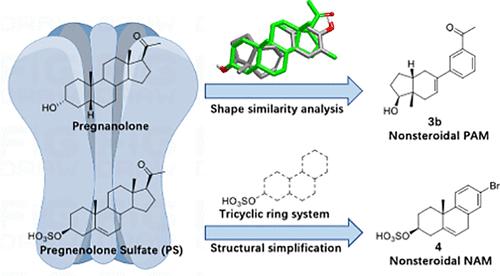
GABAA receptors (GABAARs) are the major elements of inhibitory neurotransmission in the central nervous system (CNS). They are established targets for regulation by endogenous brain neuroactive steroids (NASs) such as pregnanolone. However, the complexity of de novo synthesis of NAS derivatives has hindered attempts to circumvent the principal limitations of using endogenous NASs, including selectivity and limited oral bioavailability. In this study, we designed a series of tricyclic compounds, inspired by the structures of pregnanolone and pregnenolone sulfate, to explore novel nonsteroidal alternatives. Using patch clamp electrophysiology, we demonstrate that these compounds exhibit either positive or negative allosteric modulation of GABAARs. Specifically, we discover a positive allosteric modulator (PAM) and a series of tricyclic sulfate-based negative allosteric modulators (NAMs) all active at the micromolar level. This research has significantly broadened the chemical diversity of ligands targeting GABAARs offering potential for efficacious allosteric modulators while avoiding the complexity of NAS synthesis.
非甾体三环配体作为GABAA受体调节剂的设计、合成和药理学评价
GABAA 受体(GABAARs)是中枢神经系统(CNS)抑制性神经传递的主要成分。它们是内源性脑神经活性类固醇(NAS)(如孕烷醇酮)的既定调节靶标。然而,NAS 衍生物从头合成的复杂性阻碍了人们试图规避使用内源性 NASs 的主要局限性,包括选择性和有限的口服生物利用度。在本研究中,我们受孕烯醇酮和孕烯醇酮硫酸盐结构的启发,设计了一系列三环化合物,以探索新型非甾体类替代品。利用膜片钳电生理学,我们证明这些化合物对 GABAARs 具有正或负的异构调节作用。具体来说,我们发现了一种正性异位调节剂(PAM)和一系列基于三环硫酸盐的负性异位调节剂(NAM),它们都在微摩尔水平上具有活性。这项研究大大拓宽了以 GABAARs 为靶标的配体的化学多样性,在避免 NAS 合成的复杂性的同时,也为高效的异位调节剂提供了潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


