Isolating and stabilizing active copper species in layered double hydroxide to enhance electrocatalytic CO2 reduction to CH4
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrocatalytic CO2 reduction reaction (CO2RR) to CH4 presents an effective solution to environmental and energy challenges. Catalysts featuring monodispersed Cu sites can suppress the dimerization of *CO intermediate, which makes them promising candidates for achieving high selectivity in the deep reduction of CO2 to CH4. However, most Cu-based catalysts inevitably undergo restructuring during the reaction, which can alter the CO2 reduction pathway and result in decreased performance. In this study, a series of Cu-based layered double hydroxides (LDHs) with stable monodispersed Cu sites were developed via atom isolation strategy. Among them, the CuMgAl-LDH catalyst with the monodispersed Cu sites achieved a Faradaic efficiency (FE) of 58.9 % for CO2 reduction to CH4 at a current density of 300 mA cm−2 in a flow cell. In contrast, the CuAl-LDH catalyst without Mg doping showed a FE of 40.5 % for CO2 reduction to C2H4. The results indicate that Mg atoms can inhibit the reconstruction process of CuMgAl-LDH during working conditions, preventing the aggregation of Cu atoms, thereby maintaining a high dispersion of Cu atoms. Additionally, a pulse electrolysis regulation strategy was employed to further enhance the selectivity and stability of CuMgAl-LDH, achieving a FE of 71.6 % for CO2 reduction to CH4, with stability maintained for over 13 h. The results present a useful case for studying catalyst reconstruction and improving CO2 reduction performance.
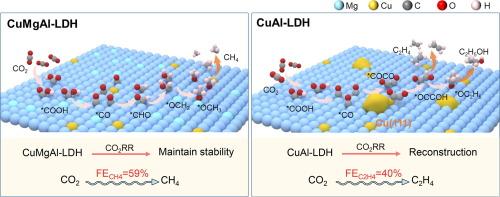
在层状双氢氧化物中分离和稳定活性铜物种,以提高电催化二氧化碳还原为甲烷的能力
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


