Insights into effects of zeolite framework topology on the cross aldol reaction of benzaldehyde with 3-Pentanone
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
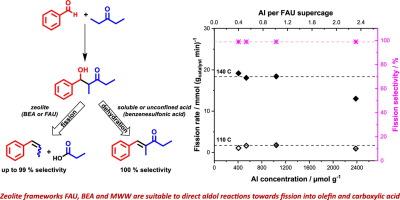
Aldol reactions are important in chemical syntheses and have high potential for use in the conversion of biomass to fuels and chemicals. Zeolites of various framework topologies (MFI, MWW, MOR, BEA, FAU) were investigated for their ability to direct aldol chemistry towards the dehydration and formation of the classical condensation product, or towards fission to an olefin and a carboxylic acid. The performance of these solid acids was benchmarked against homogeneous catalysis by benzenesulfonic acid. At reaction temperatures between 80 °C and 180 °C, autogenous pressure in toluene as the solvent, the zeolite-catalyzed cross-aldol reaction between benzaldehyde and 3-pentanone gave the fission products β-methyl-styrene and propionic acid with selectivity of up to 99 %, whereas catalysis by benzenesulfonic acid resulted in the dehydration product 2-methyl-1-phenyl-1-penten-3-one with a selectivity of 100 %. Selectivity-conversion analysis showed fission and dehydration to be parallel reactions of the intermediate ketol 1-hydroxy-2-methyl-1-phenyl-3-pentanone. Poisoning experiments with pyridine, 2,6-lutidine, triphenylphosphine and 2,4,6 tri-tert-butylpyridine demonstrate that fission is catalyzed by Brønsted acid sites and occurs in the zeolite interior of FAU and BEA, in the external cups of MWW, and to some extent in the pore mouths of all topologies. Arrhenius analysis revealed an activation energy of 107 ± 2 kJ/mol for fission in FAU. Fission selectivity in zeolites is found to be enhanced by the inability of the dehydration product to leave the pores. In the reaction-controlled regime, as determined by Weisz-Prater analysis and inspection of the Arrhenius plot, the fission rate is independent of the site density over a wide range of compositions (Si/Al from 6 to 40 for FAU). This result is interpreted as a limitation through molecular crowding in the pores, which prevents full participation of all sites in a cage. The high yields achievable to either product at maximum conversion – up to 85 % fission or 90 % dehydration – simply by choice of catalyst make acid catalysis an attractive choice in aldol chemistry.
洞察沸石框架拓扑结构对苯甲醛与 3-戊酮的交叉醛醇反应的影响
醛醇反应在化学合成中非常重要,在将生物质转化为燃料和化学品方面具有很大的应用潜力。我们研究了各种框架拓扑结构(MFI、MWW、MOR、BEA、FAU)的沸石,以了解它们引导醛醇化学反应脱水并形成经典缩合产物,或裂变为烯烃和羧酸的能力。这些固体酸的性能以苯磺酸的均相催化为基准。在反应温度为 80 ℃ 至 180 ℃、以甲苯为溶剂的常压条件下,沸石催化的苯甲醛和 3-戊酮之间的交醛反应生成了裂变产物 β-甲基苯乙烯和丙酸,选择性高达 99%,而苯磺酸催化的脱水产物为 2-甲基-1-苯基-1-戊烯-3-酮,选择性为 100%。选择性转化分析表明,裂变和脱水是中间酮醇 1-羟基-2-甲基-1-苯基-3-戊酮的平行反应。用吡啶、2,6-丁啶、三苯基膦和 2,4,6 三叔丁基吡啶进行的中毒实验表明,裂变是由布氏酸位点催化的,发生在 FAU 和 BEA 的沸石内部、MWW 的外杯以及所有拓扑结构的孔口(在一定程度上)。Arrhenius 分析显示,FAU 中裂变的活化能为 107 ± 2 kJ/mol。由于脱水产物无法离开孔隙,沸石中的裂变选择性得以提高。根据 Weisz-Prater 分析和阿伦尼乌斯曲线图的检验,在反应控制体系中,裂变率在很大的组成范围内(FAU 的硅/铝范围为 6 至 40)与位点密度无关。这一结果被解释为孔隙中的分子拥挤造成的限制,它阻碍了笼子中所有位点的充分参与。只需选择催化剂,就能在最大转化率下获得高产率(裂变率高达 85% 或脱水率高达 90%),这使得酸催化成为醛醇化学中极具吸引力的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


