Meta-Analysis of Placebo-Treated Patients: Dropout Rates From Treatment in MASH Randomised Controlled Trials
Abstract
Background
Dropout is common and affects the statistical power and randomization balance of randomised controlled trials (RCTs).
Aims
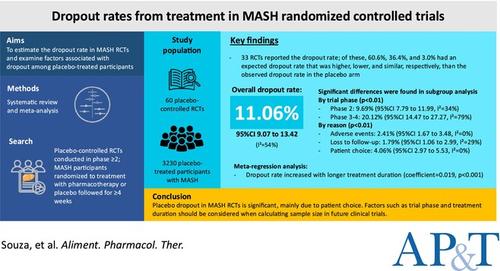
To estimate the dropout rate in RCTs of metabolic dysfunction-associated steatohepatitis (MASH) and to examine factors associated with dropout in placebo-treated participants.
Methods
PubMed and Cochrane databases were searched for phase 2–4 MASH RCTs with placebo arms through November 24, 2024. Dropout was defined as the attrition of patients included in the intention-to-treat analysis but did not complete treatment. RCTs were qualitatively reviewed to assess the expected and observed dropouts. Generalised linear mixed model was used to estimate pooled dropout rates.
Results
Sixty RCTs with 3230 placebo-treated participants with MASH were analysed. Thirty-three RCTs reported the dropout rate used to estimate the effect size. Of these, 60.6%, 36.4%, and 3.0% had an expected dropout rate that was higher, lower, and similar, respectively, than the observed dropout rate in the placebo arm. Overall, the dropout rate was 11.06% (95% confidence interval [CI] 9.07 to 13.42), with a higher rate in phase 3–4 trials than in phase 2 trials. The corresponding rates due to adverse events, loss to follow-up and patient choice were 2.41% (95% CI 1.67 to 3.48), 1.79% (95% CI 1.06 to 2.99) and 4.06% (95% CI 2.97 to 5.53), respectively. Meta-regression determined that the dropout rate increased with longer treatment duration.
Conclusion
Placebo dropout in MASH RCTs is significant, mainly due to patient choice. Factors such as trial phase and treatment duration should be considered when calculating sample size in future clinical trials.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


