Reply to “Comment on ‘Self-Illuminating Nanoagonist Simultaneously Induces Dual Cell Death Pathways via Death Receptor Clustering for Cancer Therapy’”
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
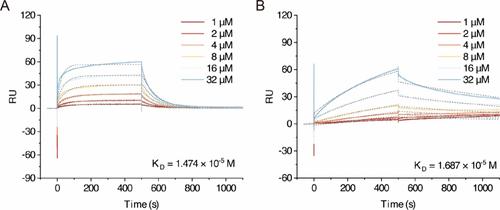
Figure 1. Sensorgrams showing the interaction between the ligand peptide and (A) human or (B) mouse DR5 at various concentrations. The fitted curves represent the kinetic analysis using a 1:1 Langmuir binding model. Multivalency effect: The conjugation of ligand peptides to the lipid-polymer hybrid OVE nanoparticles enabled the presentation of multiple peptides on the nanoparticle surface, allowing simultaneous interaction with DR5 and therefore enhancing overall binding strength. (7) Consequently, while the single peptide already demonstrated notable affinity, the multivalent nanoagonist further amplified this interaction, as evidenced by the enhanced antitumor efficacy observed in murine models (L–PEG-NP group). Optimization of flexibility and spatial presentation through PEG chains: The inclusion of 4-arm PEG in our nanoagonist design contributed to its flexibility and spatial adaptability. The high hydrophilicity and extended molecular chains of PEG allowed the ligand peptides to adopt dynamic conformations and orientations favorable for receptor binding. Unlike rigid structural scaffolds, this flexibility likely enhanced receptor engagement by enabling the peptides to adapt to the spatial and structural features of mouse DR5. (8) This spatial optimization provided by PEG and the nanoparticle scaffold facilitated even more effective interaction with mouse DR5, building upon the already significant intrinsic affinity of the ligand peptides in their monovalent form. Increased local concentration of ligand peptides: The local concentration of ligand peptides was significantly increased on the nanoparticle surface, creating a high local concentration of binding sites. This local enrichment enhanced the likelihood of interactions with mouse DR5, resulting in more frequent and stronger binding, (9) further enhancing the affinity for the murine receptor. This article references 9 other publications. This article has not yet been cited by other publications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


