Bisphenol A/Bisphenol F mineralization in the presence of self-decorated carbon-QDs@Bi2O2CO3/Ti3C2/g-C3N4 nanocomposites under multi-frequency ultrasound assisted sonophotocatalysis
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Herein, we attempted to synthesize a novel, highly efficient, surface-modified nanocatalyst supported by emerging 2D materials such as MXene (Ti3C2). A Bi2O2CO3/Ti3C2/g-C3N4 ternary Z-scheme nanostructure tethered with self-decorated Carbon Quantum Dots (C-QDs) was successfully synthesized via a simple hydrothermal method. The study presents a pioneering attempt of multi-frequency ultrasound assisted sonophotocatalysis (MFASP) for the catalytic degradation of bisphenols. The surface morphology and various contours of pristine and nanocomposites were confirmed through various analytical techniques. The decorated C-QDs, ranging from 6 to 8 nm in size were uniformly distributed across the surface of the resulting nanocomposites. The sonophotocatalytic efficiency for BPA degradation was evaluated under various operational conditions, including catalytic dosage, combinational frequencies, and nanocatalyst variations. Notably, under 20 + 40 + 80 kHz sonophotocatalytic conditions, the C-QDs@Bi2O2CO3/Ti3C2/g-C3N4 catalyst achieved 80 % degradation of BPA within 90 min (k = 0.0180 min−1). The comparison studies revealed that the order BPA elimination at MFASP was BTG > BGC > BOC > TiO2 > BMC > g-C3N4 > Ti2C3. The enhanced production of hydroxyl radicals in multi-frequency conditions was confirmed via terephthalic acid dosimetry method. Additionally, an attempt was conducted under optimized MFASP conditions for the simultaneous removal of BPA and BPF. Ultimately, more than 16 degradation intermediates proceeded through 4 different pathways (ring rupture, decarboxylation, dehydroxylation, bis-benzene ring cleavage) were identified by HRMS/QToFMS analysis, and a plausible decontamination mechanism was proposed.
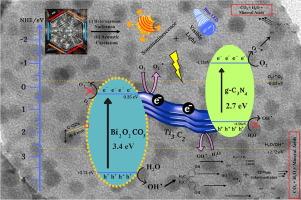
自修饰carbon-QDs@Bi2O2CO3/Ti3C2/g-C3N4纳米复合材料在多频超声辅助声光催化下的双酚A/双酚F矿化
在此,我们试图合成一种新型的、高效的、表面改性的纳米催化剂,该催化剂由新兴的二维材料如MXene (Ti3C2)支撑。采用简单的水热法成功地合成了一种自修饰碳量子点(C-QDs)系缚Bi2O2CO3/Ti3C2/g-C3N4三元Z-scheme纳米结构。本研究提出了多频超声辅助声光催化(MFASP)催化降解双酚类化合物的开创性尝试。通过各种分析技术确定了原始和纳米复合材料的表面形貌和各种轮廓。修饰后的C-QDs尺寸在6 ~ 8 nm之间,均匀分布在纳米复合材料表面。在不同的操作条件下,包括催化剂量、组合频率和纳米催化剂的变化,对双酚a降解的声光催化效率进行了评价。值得注意的是,在20 + 40 + 80 kHz sonophotocatalytic条件,C-QDs@Bi2O2CO3 / Ti3C2 / g-C3N4催化剂达到80 %退化的BPA在90 最小(0.0180 k = 分钟−1)。对比研究表明,MFASP对BPA的消除顺序为BTG和gt;BGC祝辞中行在二氧化钛 祝辞 BMC祝辞g-C3N4 祝辞 Ti2C3。通过对苯二甲酸剂量法证实了多频条件下羟基自由基的生成增强。此外,还尝试在优化的MFASP条件下同时去除BPA和BPF。最终,通过HRMS/QToFMS分析鉴定出16个以上的降解中间体通过4种不同的途径(环断裂、脱羧、去羟基化、双苯环裂解)进行降解,并提出了一种合理的去污染机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


