Baicalin attenuates the immune escape of oral squamous cell carcinoma by reducing lactate accumulation in tumor microenvironment
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
The acidic microenvironment caused by excessive lactate accumulation could inhibit immune lymphocytes antitumor activity and promote the immune escape of tumor cells. Baicalin is an active flavonoid isolated from Scutellaria baicalensis Georgi, a traditional Chinese medicinal herb with antioxidant and anti-inflammatory properties.Objectives
The present study aims to investigate whether and how baicalin inhibits oral squamous cell carcinoma (OSCC) acidic microenvironment and attenuates immune escape.Methods
Baicalin was dose-dependently administrated to OSCC cells (0–50 μmol/L). Co-culture system was constructed by OSCC cells and activated PBMCs. The proliferation and migration of OSCC cells were tested by CCK-8, colony formation, EdU, transwell assays. The cytokines were tested by ELISA kits. Mechanistical exploration was verified by RNA immunoprecipitation (RIP), fluorescence in situ hybridization (FISH) and RNA stability assays.Results
Results indicated that baicalin dose-dependently repressed the proliferation and migration of OSCC cells, and strengthened the antitumor immune activity of activated PBMCs to OSCC cells. Moreover, baicalin repressed the lactate accumulation, acidification and m6A modification level of OSCC cells. Molecular docking and MeRIP-Seq revealed that baicalin targeted LDHA via m6A-IGF2BP3-dependent manner to reduce lactate accumulation and PD-L1 expression in co-culture microenvironment.Conclusion
This study revealed the anti-tumor activity of baicalin for OSCC by reducing lactate accumulation and attenuating the immune escape in tumor microenvironment, which provided a novel insight to improve our understanding in the treatment of traditional Chinese medicine on human cancer.
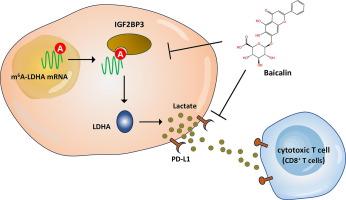
黄芩苷通过减少口腔鳞状细胞癌微环境中乳酸的积累而减弱其免疫逃逸
过量乳酸积累引起的酸性微环境可抑制免疫淋巴细胞抗肿瘤活性,促进肿瘤细胞的免疫逃逸。黄芩苷是从黄芩中分离得到的一种活性类黄酮,黄芩是一种具有抗氧化和抗炎作用的传统中药。目的探讨黄芩苷对口腔鳞状细胞癌(OSCC)酸性微环境的抑制作用及对免疫逃逸的抑制作用。方法黄芩苷以0 ~ 50 μmol/L剂量依赖性给药于OSCC细胞。将OSCC细胞与活化的pbmc细胞构建共培养体系。采用CCK-8、集落形成、EdU、transwell法检测OSCC细胞的增殖和迁移。采用ELISA试剂盒检测细胞因子。通过RNA免疫沉淀(RIP)、荧光原位杂交(FISH)和RNA稳定性实验验证了机制探索。结果黄芩苷呈剂量依赖性地抑制OSCC细胞的增殖和迁移,增强活化pbmc对OSCC细胞的抗肿瘤免疫活性。黄芩苷对OSCC细胞乳酸积累、酸化及m6A修饰水平均有抑制作用。分子对接和MeRIP-Seq分析显示,黄芪苷通过m6a - igf2bp3依赖的方式靶向LDHA,减少共培养微环境中乳酸积累和PD-L1的表达。结论黄芩苷通过降低肿瘤微环境中乳酸的积累和免疫逃逸,对OSCC具有抗肿瘤活性,为提高我们对中药治疗人类肿瘤的认识提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


