Chain Effect-controlled Solvation Chemistry and Interfacial Microstructure Enables Highly Reversible Zn Metal Anode
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
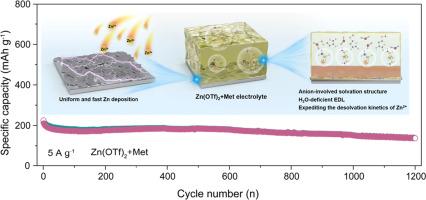
The charge transfer kinetics of Zn plating/stripping and the parasitic reactions are affected by the adsorption of Zn2+ and the subsequent desolvation process proceeded at the electrode/electrolyte interface (EEI). Herein, this work cleverly utilizes the “chain effect” triggered by the L-Methionine (Met) molecules to improving the stability of EEI by the solvation chemistry and interfacial microstructure reconfiguration. Firstly, the introduction of Met molecules enhances the solvation ability of anions, which squeezes out solvated H2O molecules and generate anion-derived hybrid solid electrolyte interphase (SEI) layer, weakening H2O-induced parasitic reactions and expediting the migration rate of Zn2+ at EEI. Meanwhile, the strengthened anion-cation interaction quickens the desolvation kinetics of Zn2+, homogenizing Zn2+ flux at interface. Secondly, Met molecules with multiple active sites not only break the original H-bonds network between H2O molecules, further reducing the reactivity of H2O molecules, but also preferentially adsorb on the Zn(101) and Zn(110) crystal planes to increase the exposure of Zn(002) crystal face, improving the stability of SEI and inducing uniform Zn deposition. Consequently, the symmetric/asymmetric cell in the Met-containing electrolyte demonstrates a long cycling life over 2700 h and high reversibility up to 4500 cycles, respectively. And the assembled full cells and pouch-cell exhibit outstanding cycling performance.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


