Sm1-XSrxMnO3 (X = 0.1, 0.2, 0.3, and 0.4) Perovskite (SSM) with A-Site Doping Optimized as Oxygen Reduction Reaction (ORR) Electrocatalyst
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
A highly selective, stable and efficient non-precious metal catalyst is crucial for alkaline fuel cell applications. Recently, rare earth-based perovskite showed improved oxygen reduction reaction (ORR) which is analogous to commercial Pt/C. In this paper, strontium doped SmMnO3 (Sm1-xSrxMnO3 (x=0.1, 0.2, 0.3, and 0.4)) are synthesized by sol-gel route. The structure, valence state, and morphology of these samples were analyzed and oxygen adsorption behavior was investigated. Further, using the RRDE electrode (rotating ring disk electrode) the electro-catalytic behavior of the prepared catalyst toward ORR were investigated. The absorption of molecular oxygen by SmMnO3 catalyst was improved and the Mn valence state was tailored by doping Sr into the crystal lattice of SmMnO3. Sm1-xSrxMnO3 (x = 0.3) displayed highest ORR activity and stability among the synthesized electrocatalyst, with kinetic current density and onset potentials of 1.15 mA/cm2, and 0.92 V, respectively. High stability and electron transfer number around 4 makes the prepared electrocatalyst a viable alternate to commercial Pt/C for alkaline fuel cell application.
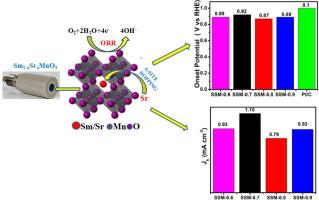
优化为氧还原反应 (ORR) 电催化剂的掺杂 A 盐的 Sm1-XSrxMnO3(X = 0.1、0.2、0.3 和 0.4)包晶 (SSM)
高选择性、稳定和高效的非贵金属催化剂对于碱性燃料电池的应用至关重要。最近,基于稀土的过氧化物显示出与商用铂/钴类似的更好的氧还原反应(ORR)。本文采用溶胶-凝胶路线合成了掺杂锶的 SmMnO3(Sm1-xSrxMnO3(x=0.1、0.2、0.3 和 0.4))。分析了这些样品的结构、价态和形貌,并研究了氧气吸附行为。此外,还使用 RRDE 电极(旋转环盘电极)研究了所制备催化剂对 ORR 的电催化行为。通过在 SmMnO3 晶格中掺入 Sr,改善了 SmMnO3 催化剂对分子氧的吸收,并调整了 Mn 的价态。在合成的电催化剂中,Sm1-xSrxMnO3(x = 0.3)具有最高的 ORR 活性和稳定性,其动力学电流密度和起始电位分别为 1.15 mA/cm2 和 0.92 V。高稳定性和 4 左右的电子转移数使所制备的电催化剂成为碱性燃料电池应用中商用 Pt/C 的可行替代品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


