Nuclear adenine activates hnRNPA2B1 to enhance antibacterial innate immunity
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
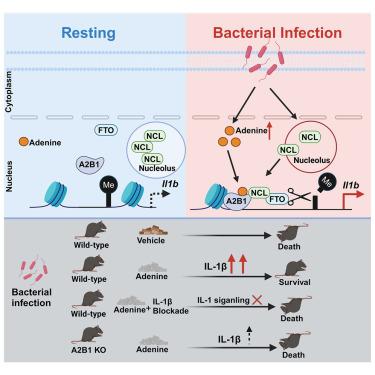
Bacterial infection reprograms cellular metabolism and epigenetic status, but how the metabolic-epigenetic crosstalk empowers host antibacterial defense remains unclear. Here, we report that heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) is a sensor for metabolite adenine to launch an antimicrobial innate response through increasing Il1b transcription. Myeloid cell-specific Hnrnpa2b1-cKO mice are more susceptible to bacterial infection, while interleukin 1 beta (IL-1β) supplementation rescues the phenotype. Through a large-scale metabolites-hnRNPA2B1 interaction screen, we reveal that adenine directly binds and activates hnRNPA2B1 to mediate innate antibacterial response. Mechanistically, adenine directly recruits hnRNPA2B1 to Il1b enhancers, and hnRNPA2B1 increases Il1b enhancer chromatin accessibility through binding and recruiting nucleolin and fat mass and obesity-associated protein (FTO) to mediate Il1b enhancer DNA N6-methyladenosine (6mA) demethylation. Furthermore, bacterial infection elevates nuclear adenine at the early stage of infection, and in vivo adenine administration protects mice from death upon bacterial infection through the hnRNPA2B1-IL-1β circuit. Our findings offer new insights into metabolic-epigenetic crosstalk relevant to antibacterial innate immunity and indicate potential approaches for treating bacterial infections.
核腺嘌呤激活 hnRNPA2B1 增强抗菌先天免疫力
细菌感染重新编程细胞代谢和表观遗传状态,但代谢-表观遗传串扰如何增强宿主抗菌防御尚不清楚。在这里,我们报道了异质核核糖核蛋白A2B1 (hnRNPA2B1)是代谢物腺嘌呤的传感器,通过增加Il1b的转录来启动抗菌先天反应。髓细胞特异性Hnrnpa2b1-cKO小鼠更容易受到细菌感染,而补充白细胞介素1β (IL-1β)可以挽救这种表型。通过大规模代谢物-hnRNPA2B1相互作用筛选,我们发现腺嘌呤直接结合并激活hnRNPA2B1介导先天抗菌反应。在机制上,腺嘌呤直接将hnRNPA2B1招募到Il1b增强子,hnRNPA2B1通过结合和招募核蛋白和脂肪质量和肥胖相关蛋白(FTO)来介导Il1b增强子DNA n6 -甲基腺苷(6mA)去甲基化,从而增加Il1b增强子染色质的可及性。此外,细菌感染在感染早期提高核腺嘌呤水平,体内腺嘌呤通过hnRNPA2B1-IL-1β通路保护小鼠免受细菌感染的死亡。我们的发现为抗菌先天免疫相关的代谢-表观遗传串扰提供了新的见解,并指出了治疗细菌感染的潜在方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


