The spatial zonation of the murine placental vasculature is specified by epigenetic mechanisms
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The labyrinthian fetoplacental capillary network is vital for proper nourishment of the developing embryo. Dysfunction of the maternal-fetal circulation is a primary cause of placental insufficiency. Here, we show that the spatial zonation of the murine placental labyrinth vasculature is controlled by flow-regulated epigenetic mechanisms. Spatiotemporal transcriptomic profiling identified a gradual change in the expression of epigenetic enzymes, including the de novo DNA methyltransferase 3a (DNMT3A). Loss of Dnmt3a resulted in DNA hypomethylation and perturbation of zonated placental gene expression. The resulting global DNA hypomethylation impaired the angiogenic capacity of endothelial cells. Global or endothelium-predominant deletion of Dnmt3a resulted in impaired placental vascularization and fetal growth retardation (FGR). Human placental endothelial gene expression profiling associated preeclampsia with reduced DNMT3A expression. Collectively, our study identified DMNT3A as critical methylome-regulator of placental endothelial gene expression and function with clinical implications for placental dysfunction, as it occurs during preeclampsia or FGR.
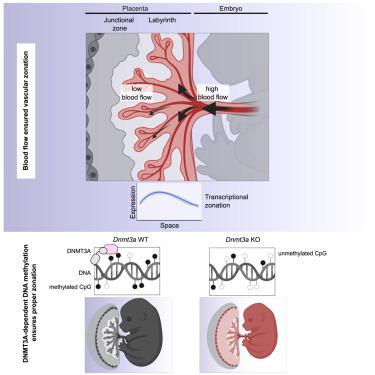
小鼠胎盘血管的空间分区由表观遗传机制决定
迷宫式胎盘毛细血管网对发育中胚胎的正常营养至关重要。母胎循环功能障碍是胎盘功能不全的主要原因。在这里,我们发现小鼠胎盘迷宫血管的空间分区是由流动调节的表观遗传机制控制的。时空转录组分析发现了表观遗传酶表达的渐变,包括DNA甲基转移酶3a(DNMT3A)。Dnmt3a的缺失导致了DNA低甲基化和带状胎盘基因表达的紊乱。由此导致的DNA整体低甲基化损害了内皮细胞的血管生成能力。Dnmt3a的全局性或内皮细胞主导性缺失会导致胎盘血管形成受损和胎儿生长迟缓(FGR)。人类胎盘内皮基因表达谱分析显示,先兆子痫与 DNMT3A 表达减少有关。总之,我们的研究确定了 DMNT3A 是胎盘内皮基因表达和功能的关键甲基组调节器,对子痫前期或 FGR 期间发生的胎盘功能障碍具有临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


