Hydrogen Bonding-Driven Adaptive Coacervates as Protocells
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Coacervation based on liquid–liquid phase separation (LLPS) has been widely used for the preparation of artificial protocells and to mimic the dynamic organization of membrane-free organelles. Most complex synthetic coacervates are formed through electrostatic interactions but cannot withstand high ionic strength conditions (>0.1 M). Alternative components and driving forces are highly desired for the formation of natural organelles to overcome the drawbacks of traditional coacervates. Herein, hydrogen bonding-driven adaptive coacervates are reported via the complexation of poly(ethylene glycol) (PEG) and tannic acid (TA). The LLPS behavior of these adaptive coacervates is dependent on the concentration and mass ratio of PEG and TA, which can be used to tune the size of coacervates ranging from 70 nm to 10 μm as well as the morphology of isotropic particles and hollow capsules. Coacervates are stable at high ionic concentrations up to 1 M and can serve as protocells to mimic cellular behaviors including metabolism (e.g., nutrient uptake), phagocytosis, and membrane fusion. The reported approach provides a platform for the rational design of hydrogen bonding-driven coacervates with controllable size and morphology, offering potential applications in protocell construction and therapeutic delivery.
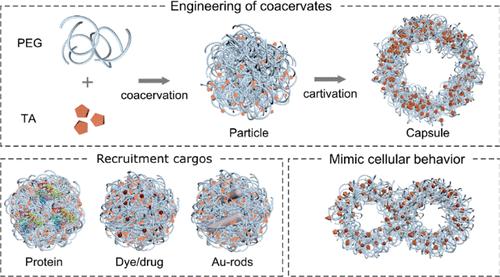
氢键驱动的适应性凝聚体作为原始细胞
基于液-液相分离(LLPS)的凝聚已被广泛应用于人工原始细胞的制备和模拟无膜细胞器的动态组织。大多数复杂的合成凝聚体是通过静电相互作用形成的,但不能承受高离子强度条件(>0.1 M)。为了克服传统凝聚体的缺点,迫切需要替代成分和驱动力来形成天然细胞器。本文报道了通过聚乙二醇(PEG)和单宁酸(TA)的络合,氢键驱动的自适应凝聚物。这些自适应凝聚体的LLPS行为取决于PEG和TA的浓度和质量比,可用于调整凝聚体的尺寸,范围从70 nm到10 μm,以及各向同性颗粒和空心胶囊的形态。凝聚体在高达1m的高离子浓度下是稳定的,可以作为原始细胞来模拟细胞行为,包括代谢(例如,营养摄取)、吞噬和膜融合。该方法为合理设计具有可控大小和形态的氢键驱动凝聚体提供了平台,在原始细胞构建和治疗递送方面具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


