Elevated GABAergic neurotransmission prevents chronic intermittent ethanol induced hyperexcitability of intrinsic and extrinsic inputs to the ventral subiculum of female rats
IF 3.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
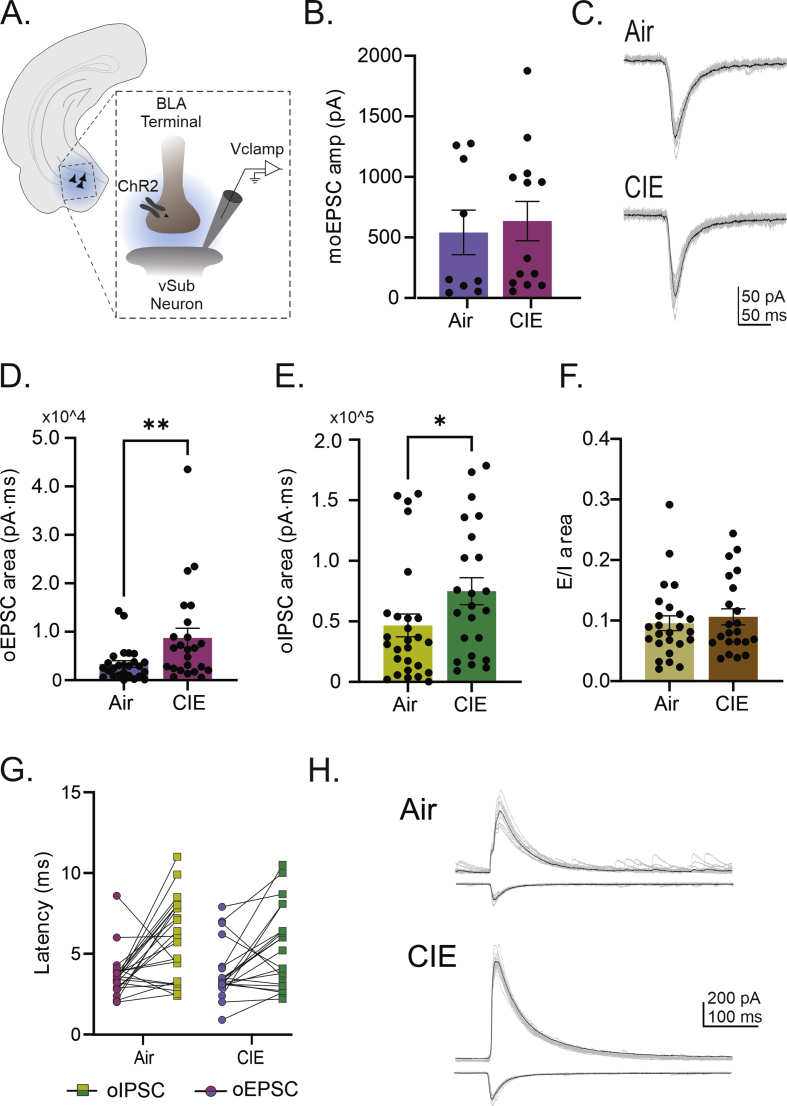
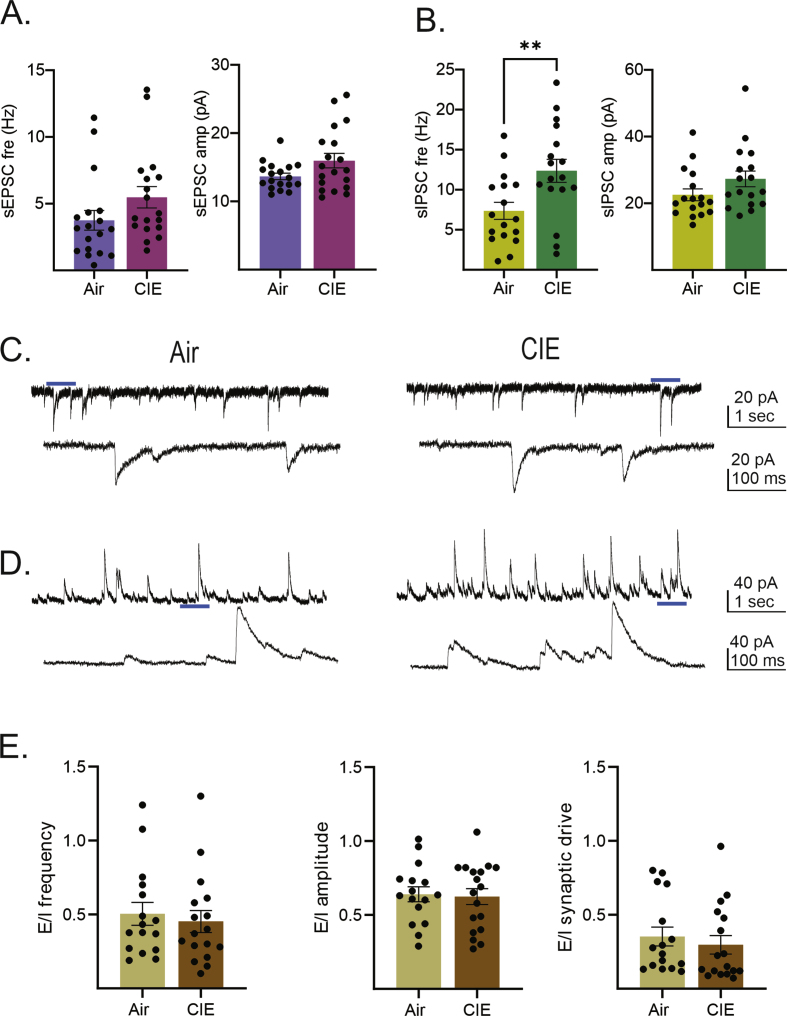
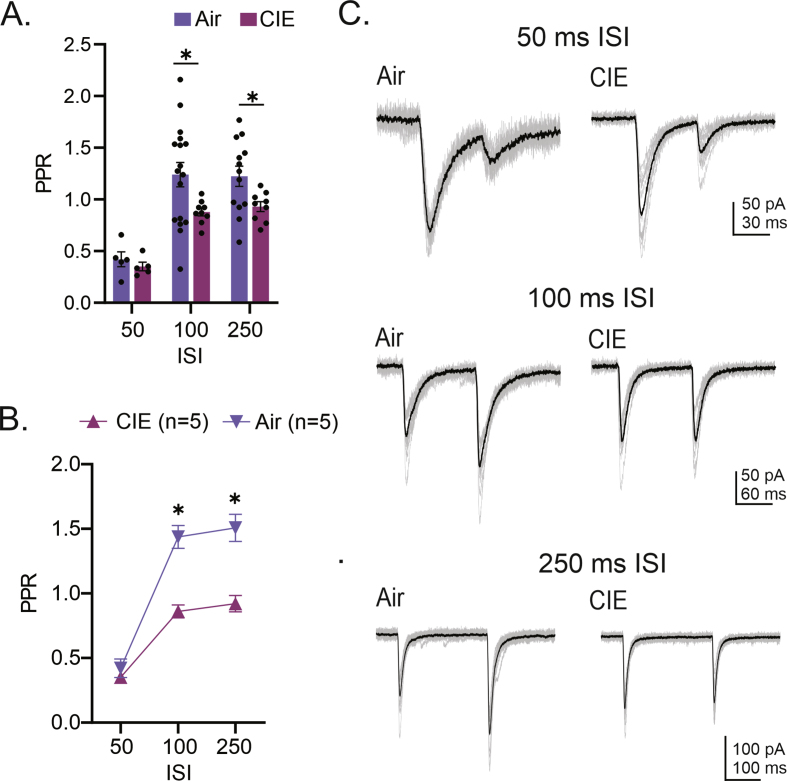
With the recent rise in the rate of alcohol use disorder (AUD) in women, the historical gap between men and women living with this condition is narrowing. While there are many commonalities in how men and women are impacted by AUD, an accumulating body of evidence is revealing sex-dependent adaptations that may require distinct therapeutic approaches. Preclinical rodent studies are beginning to shed light on sex differences in the effects of chronic alcohol exposure on synaptic activity in a number of brain regions. Prior studies from our laboratory revealed that, while withdrawal from chronic intermittent ethanol (CIE), a commonly used model of AUD, increased excitability in the ventral hippocampus (vHC) of male rats, this same treatment had the opposite effect in females. A follow-up study not only expanded on the synaptic mechanisms of these findings in male rats, but also established a CIE-dependent increase in the excitatory-inhibitory (E-I) balance of a glutamatergic projection from the basolateral amygdala to vHC (BLA-vHC). This pathway modulates anxiety-like behavior and could help explain the comorbid occurrence of anxiety disorders in individuals suffering from AUD. The present study sought to conduct a similar analysis of CIE effects on both synaptic mechanisms in the vHC and adaptations in the BLA-vHC pathway of female rats. Our findings indicate that CIE increases the strength of inhibitory neurotransmission in the vHC and that this sex-specific adaptation blocks, or at least delays, the increases in intrinsic vHC excitability and BLA-vHC synaptic transmission observed in males. Our findings establish the BLA-vHC pathway and the vHC as important circuitry to consider for future studies directed at identifying sex-dependent therapeutic approaches to AUD.
升高的gaba能神经传递可防止慢性间歇乙醇诱导的雌性大鼠腹侧下背内外输入的高兴奋性。
随着最近女性酒精使用障碍(AUD)率的上升,患有这种疾病的男性和女性之间的历史差距正在缩小。虽然男性和女性受到AUD影响的方式有许多共同点,但越来越多的证据表明,性别依赖的适应可能需要不同的治疗方法。临床前啮齿类动物研究开始揭示慢性酒精暴露对大脑许多区域突触活动影响的性别差异。我们实验室之前的研究表明,虽然从慢性间歇性乙醇(CIE)中退出(一种常用的AUD模型)会增加雄性大鼠腹侧海马(vHC)的兴奋性,但同样的治疗在雌性大鼠中却有相反的效果。一项后续研究不仅在雄性大鼠中扩展了这些发现的突触机制,而且还建立了从基底外侧杏仁核到vHC (BLA-vHC)的谷氨酸能投射的兴奋-抑制(E-I)平衡依赖于cie的增加。这一通路调节焦虑样行为,有助于解释AUD患者焦虑障碍的共病发生。本研究试图对CIE对雌性大鼠vHC突触机制和BLA-vHC通路适应性的影响进行类似的分析。我们的研究结果表明,CIE增加了vHC中抑制性神经传递的强度,这种性别特异性适应阻断或至少延迟了在男性中观察到的vHC内在兴奋性和BLA-vHC突触传递的增加。我们的研究结果确定了BLA-vHC通路和vHC是未来研究中重要的回路,这些研究旨在确定AUD的性别依赖性治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Stress
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
9.40
自引率
4.00%
发文量
74
审稿时长
48 days
期刊介绍:
Neurobiology of Stress is a multidisciplinary journal for the publication of original research and review articles on basic, translational and clinical research into stress and related disorders. It will focus on the impact of stress on the brain from cellular to behavioral functions and stress-related neuropsychiatric disorders (such as depression, trauma and anxiety). The translation of basic research findings into real-world applications will be a key aim of the journal.
Basic, translational and clinical research on the following topics as they relate to stress will be covered:
Molecular substrates and cell signaling,
Genetics and epigenetics,
Stress circuitry,
Structural and physiological plasticity,
Developmental Aspects,
Laboratory models of stress,
Neuroinflammation and pathology,
Memory and Cognition,
Motivational Processes,
Fear and Anxiety,
Stress-related neuropsychiatric disorders (including depression, PTSD, substance abuse),
Neuropsychopharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


