Development, analysis, and effectiveness of an F-C-MgO/rGOP catalyst for the degradation of atrazine using ozonation process: Synergistic effect, mechanism, and toxicity assessment
IF 8.4
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
This study considered the effects of fluoride, MgO, sucrose, and rGO on the characteristics of the fluoride-carbon-MgO/rGO predicted (F-C-MgO/rGOP) catalyst and its effectiveness in the catalytic ozonation process (COP) for atrazine elimination from aqueous solutions. Using a mixture design, the catalyst composition was optimized to 13.6% sucrose, 50% Mg (OH)2, 25% NaF, and 11.4% rGO, which demonstrated the highest catalytic activity for atrazine degradation. Analysis of the synthesized F-C-MgO/rGO revealed a mesoporous structure with a BET surface area of 145 m2/g. The optimized COP parameters were a pH of 8.5, contact time of 11 min, catalyst dose of 1.38 g/L, and atrazine concentration of 10 mg/L. Atrazine mineralization reached 62.8% after 15 min of contact time. The COP exhibited a synergistic effect of 82.5%, a mineralization capacity of 17.75 mg/g, and a kinetic rate constant of 0.25 min⁻1 for atrazine degradation. Intermediate analysis identified alkyl oxidation, dealkylation, and dechlorination-hydroxylation as the main pathways of degradation. Biodegradability index and toxicity assessments indicated a reduction in the toxicity of treated wastewater (both synthetic and real) after COP treatment. A BOD₅/COD ratio above 0.5 in both samples indicated the wastewater was suitably biodegradable.
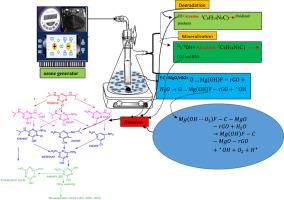
臭氧氧化法降解阿特拉津的F-C-MgO/rGOP催化剂的开发、分析和有效性:协同效应、机理和毒性评价
本研究考察了氟化物、MgO、蔗糖和rGO对氟碳-MgO/rGO预测(F-C-MgO/rGOP)催化剂特性的影响及其在催化臭氧化过程(COP)中去除水溶液中阿特拉津的效果。采用混合设计,优化后的催化剂组成为蔗糖13.6%、Mg (OH)2 50%、NaF 25%、rGO 11.4%,对阿特拉津的催化活性最高。对合成的F-C-MgO/rGO进行分析,发现其具有介孔结构,BET表面积为145 m2/g。优化后的COP参数为:pH为8.5,接触时间为11 min,催化剂用量为1.38 g/L,阿特拉津浓度为10 mg/L。接触15 min后,阿特拉津矿化率达62.8%。COP的协同效应为82.5%,矿化量为17.75 mg/g,降解阿特拉津的动力学速率常数为0.25 min - 1。中间分析确定了烷基氧化、脱烷基和脱氯-羟基化是降解的主要途径。生物可降解性指数和毒性评估表明,经过COP处理的废水(合成和真实)毒性降低。两个样品中的BOD₅/COD比均高于0.5,表明废水可适当生物降解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Management
环境科学-环境科学
CiteScore
13.70
自引率
5.70%
发文量
2477
审稿时长
84 days
期刊介绍:
The Journal of Environmental Management is a journal for the publication of peer reviewed, original research for all aspects of management and the managed use of the environment, both natural and man-made.Critical review articles are also welcome; submission of these is strongly encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


