Nano-polymeric platinum activates PAR2 gene editing to suppress tumor metastasis
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Metastasis as the hallmark of cancer preferentially contributes to tumor recurrence and therapy resistance, aggrandizing the lethality of patients with cancer. Despite their robust suppressions of tumor progression, chemotherapeutics failed to attenuate cancer cell migration and even triggered pro-metastatic effects. In parallel, protease-activated receptor 2 (PAR2), a member of the G protein-coupled receptor subfamily, actively participates in cancer metastasis via multiple signal transduction pathways. CRISPR/Cas9 that is a dominating genome editing tool can evoke PAR2 knockout to inhibit cancer metastasis. However, the absence of valid delivery systems largely limits its efficacy. Herein, we nanosized polymeric platinum (NanoPt) as therapeutical drug carries to deliver CRISPR/Cas9 to elicit genome editing of PAR2, which drastically augmented anti-metastatic effects and alleviated systematic toxicity of platinum-based treatment in vitro and in vivo. More importantly, the NanoPt@Cas9-PAR2 initiated PAR2 deficiency to mechanistically attenuate EMT process and ferroptosis via RAGE/ERK signalling, consequently preventing cancer cell migration. Our findings indicate that NanoPt@Cas9-PAR2 that mitigated PAR2 signalling and cytotoxic effects of platinum could be a safe and powerful all-in-one combinatorial strategy for cancer treatment.
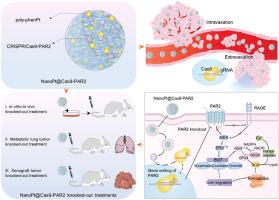
纳米聚合铂激活PAR2基因编辑抑制肿瘤转移
转移作为癌症的标志,优先导致肿瘤复发和治疗抵抗,扩大了癌症患者的死亡率。尽管化疗药物对肿瘤进展具有强大的抑制作用,但它们未能减弱癌细胞的迁移,甚至引发了促转移效应。与此同时,蛋白酶激活受体2 (PAR2)作为G蛋白偶联受体亚家族的成员,通过多种信号转导途径积极参与肿瘤转移。CRISPR/Cas9是一种主要的基因组编辑工具,可以通过敲除PAR2来抑制癌症转移。然而,缺乏有效的递送系统在很大程度上限制了其效力。本文中,我们将纳米聚合物铂(NanoPt)作为治疗药物载体,递送CRISPR/Cas9,引发PAR2的基因组编辑,从而大大增强了抗转移效果,减轻了铂基治疗的体内体外系统毒性。更重要的是,NanoPt@Cas9-PAR2启动PAR2缺陷,通过RAGE/ERK信号传导机制减弱EMT过程和铁凋亡,从而阻止癌细胞迁移。我们的研究结果表明,NanoPt@Cas9-PAR2减轻了铂的PAR2信号传导和细胞毒性作用,可能是一种安全有效的癌症治疗一体化组合策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


