SHED-Derived Exosomes Ameliorate Sjögren’s Syndrome-Induced Hyposalivation by Suppressing Th1 Cell Response via the miR-29a-3p/T-bet Axis
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Background: Sjögren’s syndrome (SS), an autoimmune disease, was characterized by sicca syndrome and systemic manifestations, presenting significant treatment challenges. Exosomes, naturally derived nanoparticles containing bioactive molecules, have garnered interest in regenerative medicine. The present study aimed to elucidate the immunoregulatory properties and mechanism of exosomes obtained from the stem cells derived from human exfoliated deciduous teeth (SHED-exos) in SS-induced sialadenitis. Methods: SHED-exo nanoparticles were injected into submandibular glands (SMGs) of 14-week-old nonobese diabetic (NOD) mice, a classic animal model of SS. At 21 weeks, the saliva flow rate (SFR) was measured. Lymphocyte proportions were examined via flow cytometry. Inflammatory cytokine levels were examined by the Quantibody mouse Th1/Th2/Th17 array and ELISA. miR-29a-3p expression and its regulatory effect on T-bet was detected using FISH and luciferase reporter gene assay, respectively. Results: SHED-exos injected into SMGs increased SFR, reduced lymphocytic infiltration, and decreased inflammatory cytokine levels in serum, SMG tissues, and saliva. Mechanistically, SHED-exos suppressed the Th1 proportion in spleen lymphocytes in NOD mice. Exosomal miR-29a-3p targeted and suppressed T-bet expression, which is a Th1-specific transcription factor. In vitro, SHED-exos (but not miR-29a-3p-inhibited exosomes) decreased the level of Th1 differentiation and IFN-γ and TNF-α production. Furthermore, SHED-exos (but not miR-29a-3p-inhibited exosomes) blocked the increase in IFN-γ and TNF-α production induced by T-bet overexpression. In vivo, miR-29a-3p-inhibited exosomes neither increase saliva secretion in NOD mice nor decrease lymphocytic infiltration, T-bet expression, and IFN-γ and TNF-α levels in SMGs. Conclusion: SHED-exos suppress Th1 cell differentiation and response through the miR-29a-3p/T-bet axis, contributing to amelioration of SS-induced hyposalivation.
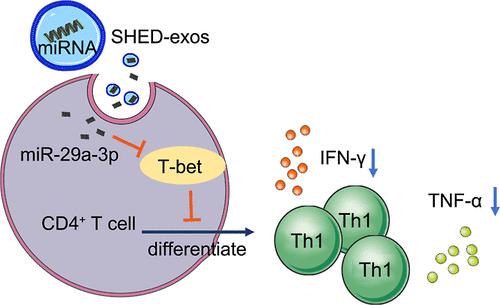
SHED衍生的外泌体通过miR-29a-3p/T-bet轴抑制Th1细胞反应,从而改善Sjögren综合征诱发的唾液分泌过少症
背景:斯约格伦综合征(SS)是一种自身免疫性疾病,以眼综合征和全身表现为特征,给治疗带来了巨大挑战。外泌体是一种天然衍生的纳米颗粒,含有生物活性分子,在再生医学中备受关注。本研究旨在阐明从人类脱落牙齿干细胞(SHED-exos)中获得的外泌体在 SS 引起的咽峡炎中的免疫调节特性和机制。研究方法将SHED-exo纳米颗粒注射到14周大的非肥胖糖尿病(NOD)小鼠的颌下腺(SMGs)中,这是一种典型的SS动物模型。21 周时,测量唾液流速(SFR)。通过流式细胞术检测淋巴细胞比例。利用 FISH 和荧光素酶报告基因测定法分别检测了 miR-29a-3p 的表达及其对 T-bet 的调控作用。结果向SMG注射SHED-exos可增加SFR,减少淋巴细胞浸润,降低血清、SMG组织和唾液中的炎症细胞因子水平。从机理上讲,SHED-exos抑制了NOD小鼠脾脏淋巴细胞中Th1的比例。外泌体 miR-29a-3p 靶向并抑制了 Th1 特异性转录因子 T-bet 的表达。在体外,SHED-exos(而不是抑制 miR-29a-3p 的外泌体)降低了 Th1 分化水平以及 IFN-γ 和 TNF-α 的产生。此外,SHED-exos(而不是 miR-29a-3p 抑制外泌体)阻止了 T-bet 过表达诱导的 IFN-γ 和 TNF-α 生成的增加。在体内,miR-29a-3p 抑制外泌体既不会增加 NOD 小鼠的唾液分泌,也不会减少 SMG 中的淋巴细胞浸润、T-bet 表达以及 IFN-γ 和 TNF-α 水平。结论SHED-exos通过miR-29a-3p/T-bet轴抑制Th1细胞的分化和反应,有助于改善SS诱导的唾液分泌过少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


