Protic Poly(ionic liquid)s with High Ionic Conductivity and Li+ Transport Number as Moderate Temperature Solid Electrolytes
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Despite the advantages over traditional liquid electrolytes, solid-state polymer electrolytes present several challenges to be addressed for their widespread application in solid-state lithium metal batteries. In this work, we report a novel class of solid polymer electrolytes based on protic poly(diallylmethylammonium) bis(fluorosulfonyl)imide (poly(DAMAH)FSI) with high lithium bis(fluorosulfonyl)imide (LiFSI) salt content. Protic polymer electrolytes with different compositions show improved properties over their aprotic counterpart. We postulate that the protic nature of the polymer backbone positively affects the coordination of the FSI anions leading to a Li-FSI-polycation cocoordination environment with resultant high Li diffusivity of 4.1 × 10–11 m2 s–1 and ionic conductivity of 6.4 × 10–4 S cm–1 at 80 °C which is a factor almost 10 times higher than the equivalent aprotic systems (0.7 × 10–4 S cm–1 at 80 °C). The apparent superior salt dissociation ability leads to homogeneous mixtures with a 1:2 mol ratio of Poly(DAMAH)FSI:LiFSI characterized by a Li+ ion transport number of 0.67. The cyclic voltammetry of the polymer materials on a Cu working electrode indicates the stability of the N–H proton in these high LiFSI-containing electrolytes, allowing successful plating/stripping analysis using a protic polymer-based electrolyte in a Li/Li cell. We thus demonstrate for the first time, the potential of these solvent-free protic poly(ionic liquid)-based solid polymer electrolytes in Li/Li metal cells at a moderate temperature of 50 °C, paving the way for future investigation and implementation in Li metal and Li-ion cells.
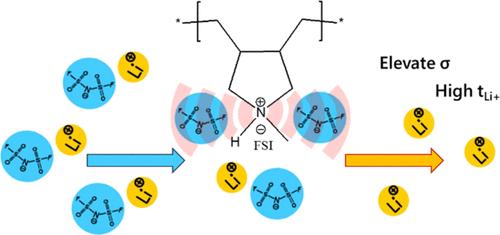
尽管固态聚合物电解质比传统液态电解质更具优势,但要在固态锂金属电池中得到广泛应用,还需应对一些挑战。在这项研究中,我们报告了一类新型固态聚合物电解质,它基于原生聚(二烯丙基甲基铵)双(氟磺酰)亚胺(poly(DAMAH)FSI)和高含量双(氟磺酰)亚胺锂盐(LiFSI)。与无色聚合物相比,不同成分的原态聚合物电解质具有更好的性能。我们推测,聚合物骨架的质子性对 FSI 阴离子的配位产生了积极影响,从而形成了锂-FSI-多阳离子共配位环境,使锂离子在 80 °C 时的扩散率高达 4.1 × 10-11 m2 s-1,离子电导率高达 6.4 × 10-4 S cm-1,比等效的非沸腾体系(80 °C 时为 0.7 × 10-4 S cm-1)高出近 10 倍。由于盐解离能力明显较强,因此聚(DAMAH)FSI:LiFSI 的摩尔比为 1:2,形成了均匀的混合物,Li+ 离子迁移数为 0.67。聚合物材料在铜工作电极上的循环伏安法表明,N-H 质子在这些高含 LiFSI 的电解质中非常稳定,因此可以在锂/锂电池中使用基于原生聚合物的电解质成功进行电镀/剥离分析。因此,我们首次证明了这些无溶剂原生聚(离子液体)基固体聚合物电解质在 50 °C 的适中温度下在锂/锂金属电池中的应用潜力,为今后在锂金属和锂离子电池中的研究和应用铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


