Predicting Shock Sensitivity from Differential Scanning Calorimetry Data and Molecular Structure: Beyond the Yoshida Correlation
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The Yoshida correlation is widely used in the pharmaceutical and fine chemical industry to predict explosivity and shock sensitivity of chemical substances based on the initiation temperature and enthalpy of differential scanning calorimetry (DSC) exotherms. We investigate the origins and accuracy of this correlation (and commonly used modifications thereof) by applying it to a large data set of 383 compounds, which are relevant to the pharmaceutical industry, and demonstrate that the initiation temperature and enthalpy variables are not good predictors for shock sensitivity. By incorporating structural information (for the 292 compounds where it was available), we used machine learning to inform and guide a logistic regression technique to develop a shock sensitivity model which has a higher overall accuracy (63%) and a higher accuracy for shock-sensitive compounds (97%) compared to the original Yoshida correlation (52% overall accuracy, 82% accuracy for shock-sensitive compounds). This logistic regression model includes both the original Yoshida variables (DSC initiation temperature and enthalpy) and also incorporates the oxygen balance (OB100) and the number of energetic nitrogen groups in the molecule.
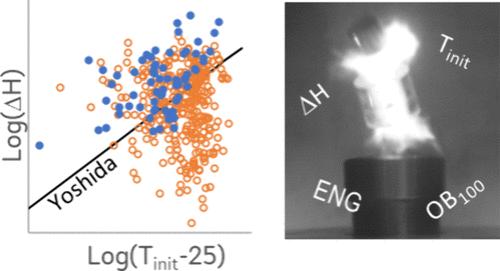
从差示扫描量热数据和分子结构预测冲击敏感性:超越吉田相关性
吉田相关法广泛应用于制药和精细化工行业,根据差示扫描量热法(DSC)放热的起始温度和焓来预测化学物质的爆炸性和冲击敏感性。我们通过将其应用于与制药工业相关的383种化合物的大型数据集,研究了这种相关性的起源和准确性(以及常用的修改),并证明了起始温度和焓变量不是冲击敏感性的良好预测因子。通过整合结构信息(对于292种可用的化合物),我们使用机器学习来告知和指导逻辑回归技术来开发冲击敏感性模型,该模型与原始的吉田相关性相比具有更高的总体精度(63%)和更高的冲击敏感性化合物精度(97%)(总体精度为52%,冲击敏感性化合物精度为82%)。该逻辑回归模型既包括原始吉田变量(DSC起始温度和焓),也包括氧平衡(OB100)和分子中含能氮基团的数量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


