Water-Compatible Staudinger–Diels–Alder Ligation
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The development of bioorthogonal reactions is expected to propel further advances in chemical biology. In this study, we demonstrate Staudinger–Diels–Alder (SDA) ligation as a candidate for a new bioorthogonal reaction. This reaction ligates two molecules via strong C–C bonds at room temperature. We found that the aryl substituent of azide-benzocyclobutene (azide-BCB) had a strong influence on the molecule’s tolerance to water. In particular, Cl-substituted azide-BCBs generated the ligated product in high yield, even in the presence of water. Mechanistic investigations using DFT methods revealed that hydrophobic electron-withdrawing substituents suppressed the side reactions of SDA ligation.
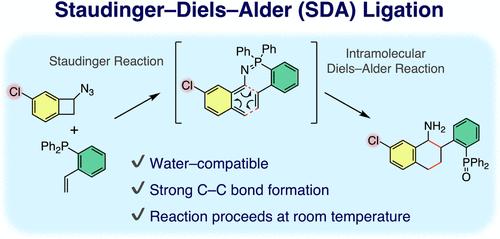
水兼容Staudinger-Diels-Alder结扎
生物正交反应的发展有望推动化学生物学的进一步进步。在本研究中,我们展示了作为新型生物正交反应候选物的 Staudinger-Diels-Alder (SDA) 连接。该反应在室温下通过强 C-C 键连接两个分子。我们发现叠氮-苯并环丁烯(叠氮-BCB)的芳基取代基对分子对水的耐受性有很大影响。尤其是 Cl 取代的叠氮-苯并环丁烯即使在有水的情况下也能以高产率生成配位产物。利用 DFT 方法进行的机理研究表明,疏水的电子吸收取代基抑制了 SDA 连接的副反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


