Rapid discovery of cyclic peptide protein aggregation inhibitors by continuous selection
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
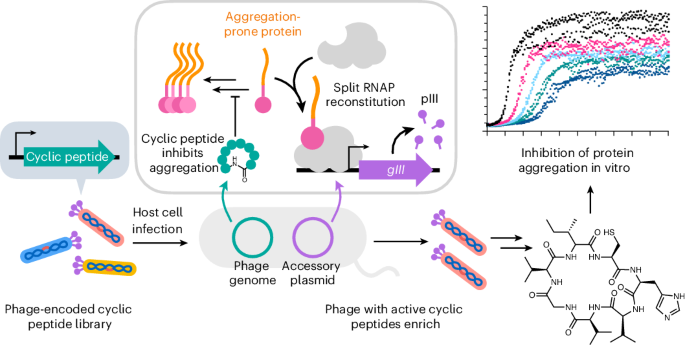
Protein aggregates are associated with numerous diseases. Here we report a platform for the rapid phenotypic selection of protein aggregation inhibitors from genetically encoded cyclic peptide libraries in Escherichia coli based on phage-assisted continuous evolution (PACE). We developed a new PACE-compatible selection for protein aggregation inhibition and used it to identify cyclic peptides that suppress amyloid-β42 and human islet amyloid polypeptide aggregation. Additionally, we integrated a negative selection that removes false positives and off-target hits, greatly improving cyclic peptide selectivity. We show that selected inhibitors are active when chemically resynthesized in in vitro assays. Our platform provides a powerful approach for the rapid discovery of cyclic peptide inhibitors of protein aggregation and may serve as the basis for the future evolution of cyclic peptides with a broad spectrum of inhibitory activities. A platform for the continuous selection of protein aggregation inhibitors from genetically encoded cyclic peptide libraries in Escherichia coli was developed. This platform was used to discover cyclic peptides that suppress aggregation of amyloid-β42 and human islet amyloid polypeptide.

通过连续选择快速发现环肽蛋白聚集抑制剂
蛋白质聚集体与许多疾病有关。在这里,我们报告了一个基于噬菌体辅助连续进化(PACE)的平台,用于从大肠杆菌遗传编码的环肽文库中快速选择蛋白质聚集抑制剂的表型。我们开发了一种新的与pace兼容的蛋白质聚集抑制选择,并使用它来鉴定抑制淀粉样蛋白-β42和人类胰岛淀粉样蛋白多肽聚集的环肽。此外,我们整合了负面选择,消除假阳性和脱靶命中,大大提高了环肽的选择性。我们表明,选定的抑制剂在体外化学合成时是有活性的。我们的平台为快速发现蛋白质聚集的环肽抑制剂提供了一种强大的方法,并可能作为具有广谱抑制活性的环肽未来进化的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


