Differential roles of human CD4+ and CD8+ regulatory T cells in controlling self-reactive immune responses
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
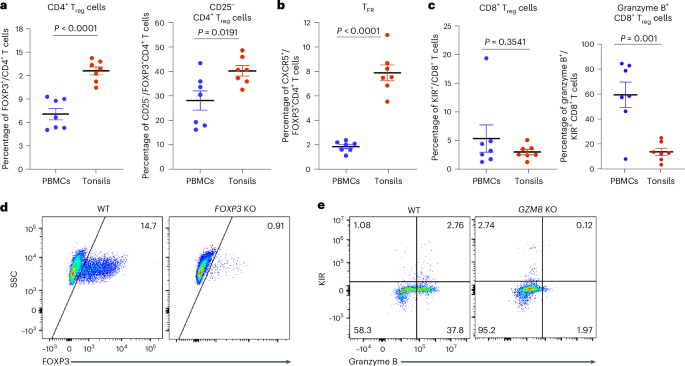
Here we analyzed the relative contributions of CD4+ regulatory T cells expressing Forkhead box protein P3 (FOXP3) and CD8+ regulatory T cells expressing killer cell immunoglobulin-like receptors to the control of autoreactive T and B lymphocytes in human tonsil-derived immune organoids. FOXP3 and GZMB respectively encode proteins FOXP3 and granzyme B, which are critical to the suppressive functions of CD4+ and CD8+ regulatory T cells. Using CRISPR–Cas9 gene editing, we were able to achieve a reduction of ~90–95% in the expression of these genes. FOXP3 knockout in tonsil T cells led to production of antibodies against a variety of autoantigens and increased the affinity of influenza-specific antibodies. By contrast, GZMB knockout resulted in an increase in follicular helper T cells, consistent with the ablation of CD8+ regulatory T cells observed in mouse models, and a marked expansion of autoreactive CD8+ and CD4+ T cells. These findings highlight the distinct yet complementary roles of CD8+ and CD4+ regulatory T cells in regulating cellular and humoral responses to prevent autoimmunity. Here the authors use a tonsil organoid culture model system to investigate the roles of human CD4+ and CD8+ regulatory T cells in controlling self-reactive immune responses. CD4+ regulatory T cells were stronger regulators of autoreactive B cells, autoantibodies and antigen-specific antibody affinity, whereas CD8+ regulatory T cells predominantly controlled expansion of follicular helper cells and autoreactive CD4+ and CD8+ T cells.

人CD4+和CD8+调节性T细胞在控制自身反应性免疫应答中的差异作用
在这里,我们分析了表达叉头盒蛋白P3 (FOXP3)的CD4+调节性T细胞和表达杀伤细胞免疫球蛋白样受体的CD8+调节性T细胞对控制人类扁桃体来源的免疫类器官中自身反应性T细胞和B淋巴细胞的相对贡献。FOXP3和GZMB分别编码FOXP3和颗粒酶B蛋白,它们对CD4+和CD8+调节性T细胞的抑制功能至关重要。使用CRISPR-Cas9基因编辑,我们能够将这些基因的表达减少约90-95%。在扁桃体T细胞中敲除FOXP3导致产生针对多种自身抗原的抗体,并增加流感特异性抗体的亲和力。相比之下,GZMB敲除导致滤泡辅助性T细胞增加,与小鼠模型中观察到的CD8+调节性T细胞的消融一致,并且自身反应性CD8+和CD4+ T细胞显著扩增。这些发现强调了CD8+和CD4+调节性T细胞在调节细胞和体液反应以预防自身免疫中的独特但互补的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


