Loss of Kmt2c or Kmt2d primes urothelium for tumorigenesis and redistributes KMT2A–menin to bivalent promoters
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
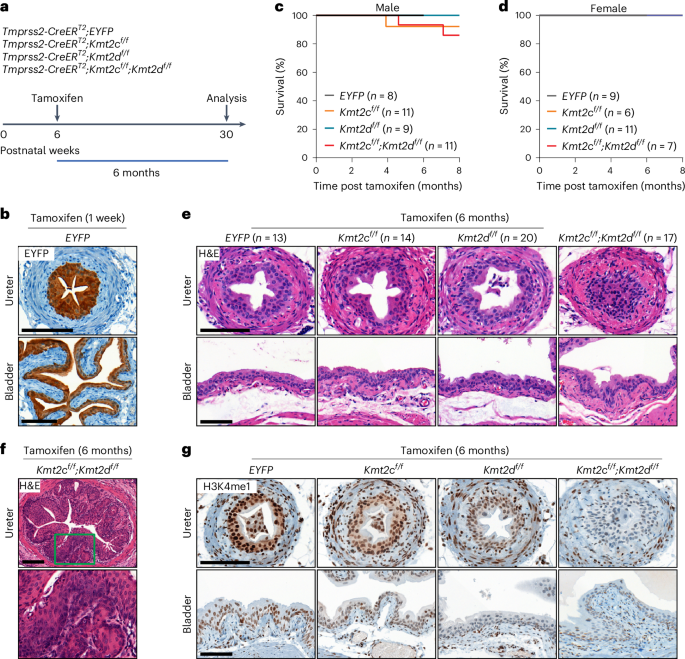
Members of the KMT2C/D–KDM6A complex are recurrently mutated in urothelial carcinoma and in histologically normal urothelium. Here, using genetically engineered mouse models, we demonstrate that Kmt2c/d knockout in the urothelium led to impaired differentiation, augmented responses to growth and inflammatory stimuli and sensitization to oncogenic transformation by carcinogen and oncogenes. Mechanistically, KMT2D localized to active enhancers and CpG-poor promoters that preferentially regulate the urothelial lineage program and Kmt2c/d knockout led to diminished H3K4me1, H3K27ac and nascent RNA transcription at these sites, which leads to impaired differentiation. Kmt2c/d knockout further led to KMT2A–menin redistribution from KMT2D localized enhancers to CpG-high and bivalent promoters, resulting in derepression of signal-induced immediate early genes. Therapeutically, Kmt2c/d knockout upregulated epidermal growth factor receptor signaling and conferred vulnerability to epidermal growth factor receptor inhibitors. Together, our data posit that functional loss of Kmt2c/d licenses a molecular ‘field effect’ priming histologically normal urothelium for oncogenic transformation and presents therapeutic vulnerabilities. Loss of Kmt2c or Kmt2d in mice drives the redeployment of KMT2A–menin to bivalent promoters, leading to changes in gene expression. This primes cells for transformation and elicits sensitivity to EGFR inhibitors.

Kmt2c或Kmt2d的缺失启动了尿路上皮的肿瘤发生,并将KMT2A-menin重新分配给二价启动子
KMT2C/D-KDM6A复合物的成员在尿路上皮癌和组织学正常的尿路上皮中反复发生突变。在这里,我们利用基因工程小鼠模型证明,Kmt2c/d 基因敲除会导致尿路细胞分化受损、对生长和炎症刺激的反应增强以及对致癌物质和致癌物质的致癌转化敏感。从机理上讲,KMT2D定位于活性增强子和CpG贫乏的启动子,它们优先调控尿路细胞系程序,Kmt2c/d基因敲除导致这些位点的H3K4me1、H3K27ac和新生RNA转录减少,从而导致分化受损。Kmt2c/d 基因敲除进一步导致 KMT2A-menin 从 KMT2D 定位的增强子重新分布到 CpG 高和二价启动子,从而导致信号诱导的即刻早期基因受到抑制。从治疗角度看,Kmt2c/d 基因敲除会上调表皮生长因子受体信号转导,并使患者易受表皮生长因子受体抑制剂的影响。综上所述,我们的数据推测,Kmt2c/d的功能性缺失会产生分子 "场效应",使组织学上正常的尿路上皮细胞发生致癌转化,并带来治疗上的脆弱性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


