High-efficiency Pb2+ removal by hydroxy-sodalite for point-of-use drinking water purification
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The development of cost-effective point-of-use (POU) devices that effectively remove lead (Pb) from drinking water is imperative in mitigating the threat of Pb contamination to public health in underdeveloped regions. Herein, we have successfully transformed inexpensive natural kaolinite as hydroxy-sodalite (HySOD) via a simple hydrothermal process, achieving an impressive yield of 91.5 %. Remarkably, HySOD demonstrates excellent selectivity and affinity towards Pb2+ with an adsorption capacity of 476 mg/g in a single Pb2+ system and a high distribution coefficient of 5.0 × 107 mL/g in multi-cations system, several orders of magnitude higher than other cations, showing remarkable Pb2+ removal efficiency. Mechanism studies reveal that the preeminent Pb2+ capture capacity of HySOD is mainly attributed to the fast surface chemisorption effects and spontaneous phase change from Na8Al6Si6O24(OH)2·2H2O to Pb4Al6Si6O24(OH)2·5H2O caused by cation exchange effects. Through a continuous filtration test, a simplified HySOD-loaded POU device is employed to treat Pb-contaminated water with the Pb2+ concentration of 200 μg/L. At a high water flux of 477 L/m2/h, the Pb2+ effluent concentration is swiftly reduced below 10 μg/L, well meeting the security standard for drinking water. Overall, this work introduces a remarkable Pb2+ removal material, showing significant application potential for POU drinking water purification.
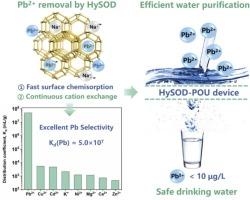
氢氧钠石高效去除Pb2+用于饮用水净化
开发具有成本效益的使用点(POU)设备,有效地从饮用水中去除铅,对于减轻不发达地区铅污染对公共健康的威胁至关重要。在此,我们成功地通过简单的水热工艺将廉价的天然高岭石转化为羟基钠石质(HySOD),收率达到了惊人的91.5%。HySOD对Pb2+表现出良好的选择性和亲和性,在单一Pb2+体系中的吸附量为476 mg/g,在多阳离子体系中的分配系数为5.0 × 107 mL/g,比其他阳离子高出几个数量级,表现出显著的Pb2+去除效率。机理研究表明,HySOD之所以具有优异的Pb2+捕获能力,主要是由于其快速的表面化学吸附效应和阳离子交换效应引起的Na8Al6Si6O24(OH)2·2H2O自发相变所致。通过连续过滤试验,采用简化的hysod负载POU装置处理Pb2+浓度为200 μg/L的铅污染水。在高水量为477 L/m2/h时,出水Pb2+浓度迅速降至10 μg/L以下,完全满足饮用水安全标准。总之,本研究介绍了一种出色的Pb2+去除材料,在POU饮用水净化中具有重要的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


