Revealing the Multifunctional Nature of Surfactant Electrolyte Additive in Aqueous Zinc Ion Batteries
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Aqueous zinc ion batteries (ZIBs) have attracted increasing attention because of their safe aqueous electrolyte, relatively low price, and suitable energy density. Lots of researchers have reported the use of surfactants as electrolyte additives to improve battery performance in AZIBs, but most of them focus on the zinc anode issues. Here, the surfactant electrolyte additive strategy was used to investigate the effect of the cathode/ electrolyte interface on cathode dissolution suppression, self-discharge inhibition, and desolvation kinetics. As a result, the electrolyte with sodium dodecyl sulfate (SDS) additive immersed with hydrated sodium vanadate electrodes shows no significant color change during the 24-hour immersion test, while the original ZnSO4 electrolyte turns yellow after only 0.5 hours. The discharge capacity of the electrolyte with SDS addition after the open-circuit voltage (OCV) test is 97.8% of the charging capacity before the OCV test, while the discharge capacity of the ZnSO4 electrolyte is only 78.7%. These results demonstrate the surfactant electrolyte additive strategy could be a feasible way to construct robust ZIBs with suppressed cathode dissolution, inhibited self-discharge, and improved interface kinetics. This work provides new insights to understand the electrolyte additive and offers a reference for other energy storage systems.
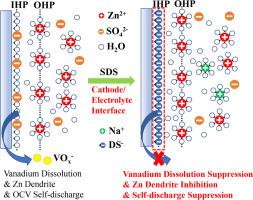
揭示锌离子电池中表面活性剂电解质添加剂的多功能性质
水锌离子电池以其安全的水电解质、相对低廉的价格和适宜的能量密度而受到越来越多的关注。许多研究人员报道了使用表面活性剂作为电解液添加剂来提高AZIBs电池性能,但大多数研究都集中在锌阳极问题上。本文采用表面活性剂电解质添加策略,研究了阴极/电解质界面对阴极溶解抑制、自放电抑制和脱溶动力学的影响。结果表明,添加十二烷基硫酸钠(SDS)添加剂的电解液与水合钒酸钠电极一起浸泡24小时后,其颜色没有明显变化,而原始的ZnSO4电解液仅在0.5小时后就变黄。开路电压(OCV)试验后添加SDS的电解液放电容量为OCV试验前充电容量的97.8%,而添加ZnSO4的电解液放电容量仅为OCV试验前充电容量的78.7%。这些结果表明,表面活性剂电解质的添加策略是一种可行的方法,可以抑制阴极溶解,抑制自放电,改善界面动力学。这项工作为理解电解质添加剂提供了新的见解,并为其他储能系统提供了参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


