Role of intermolecular interactions in the Coverage- and configuration-dependent adsorption of carboxylic acids on Pt(111)
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
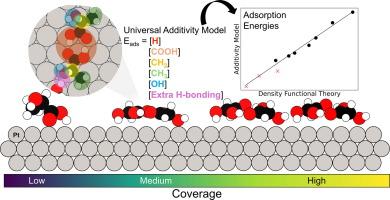
Carboxylic acids derived from biomass can be upgraded via heterogeneous catalytic processes to replacement petrochemicals or green hydrogen. A limiting factor in the catalytic upgrading of biomass-derived carboxylic acids is the varied composition of reactant mixtures and consequential competitive adsorption effects between acids that ultimately control reactivity. To address this limitation, the combined effects of intermolecular interactions and acid molecular structure on the dominant adsorbed acid configurations at catalytically relevant coverages must be explored. Here, we determine the coverage- and configuration-dependent adsorption behavior of seven carboxylic acids on Pt(111) using density functional theory and ab initio molecular dynamics simulations. The carboxylic acids—ranging from formic to lactic acid—were chosen to vary carbon chain length and terminal end substituents. The results show that at moderate to high coverages, carboxylic acids preferentially form dimers on Pt(111), regardless of the individual acid’s molecular structure. This is due to strongly attractive intermolecular interactions through hydrogen bonding between neighboring R-COOH substituents. Dimer stability was further influenced by carbon chain length and the number and chain placement of R-OH substituents. Finally, the observed trends in adsorption energy with acid molecular structure were used to develop and validate a general additivity model for predicting the adsorption energies of carboxylic acid dimers on Pt(111). This additivity model sheds light on the relative contributions of various substituents to adsorption strength: –COOH > –OH > –CH3. Overall, this work elucidates the important role of intermolecular interactions in the coverage- and configuration-dependent adsorption of carboxylic acids on transition metal surfaces. Furthermore, we provide a predictive tool for easily and rapidly rationalizing competitive adsorption effects during the catalytic upgrading of multi-component carboxylic acid mixtures.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


