Electrocatalytic CO2 Reduction to Methanol on Pt(111) Modified with a Pd Monolayer
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrochemical carbon dioxide (CO2) conversion to value-added, highly reduced chemicals such as methanol (CH3OH) is a promising possibility for producing renewable fuel and simultaneous CO2 recycling. However, this process remains a challenge, with only a few selective electrocatalysts known. Here, we present a study of a palladium monolayer on a platinum (111) single crystal (PdML/Pt(111)) as an electrocatalyst for CO2 conversion to CH3OH. A custom-made setup was employed in order to detect and quantify gaseous and liquid CO2 reduction products in sufficient concentrations despite the limitations of working with a single-crystalline electrode. Under ambient reaction conditions, a Faradaic efficiency (FE) of 1.5% at −0.9 V vs reversible hydrogen electrode (RHE) was obtained while using CO2 as the reactant. Other reaction intermediates, carbon monoxide (CO) and formaldehyde (HCHO) were subsequently used as reactants, leading to FEs of 1.8 and 2.5%, respectively, whereas formic acid is not reduced. The corresponding mechanism concluded from our work is compared to the literature. The electrocatalyst introduced here, with a highly well-defined structure for CO2 conversion to CH3OH, opens up possibilities for further catalytic explorations.
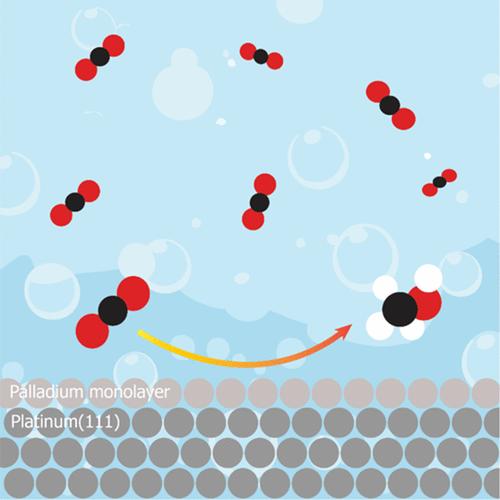
钯单分子层修饰Pt(111)上电催化CO2还原制甲醇
电化学将二氧化碳(CO2)转化为高附加值、高度还原的化学品,如甲醇(CH3OH),是生产可再生燃料和同时回收二氧化碳的一种很有前途的可能性。然而,这一过程仍然是一个挑战,只有少数选择性电催化剂已知。在这里,我们研究了铂(111)单晶(PdML/Pt(111))上的钯单层作为二氧化碳转化为CH3OH的电催化剂。尽管使用单晶电极存在局限性,但为了检测和量化足够浓度的气态和液态CO2还原产物,采用了定制的装置。在环境反应条件下,以CO2为反应物,在−0.9 V vs可逆氢电极(RHE)条件下获得了1.5%的法拉第效率(FE)。其他反应中间体,一氧化碳(CO)和甲醛(HCHO)随后被用作反应物,导致FEs分别为1.8和2.5%,而甲酸没有被还原。并与文献进行了比较。本文介绍的电催化剂具有高度明确的CO2转化为CH3OH的结构,为进一步的催化探索开辟了可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


