Rational Design of Dual-Targeted Nanomedicines for Enhanced Vascular Permeability in Low-Permeability Tumors
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
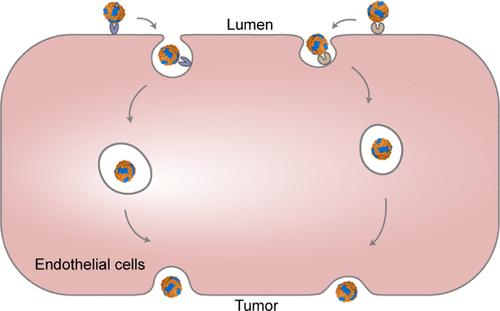
Designing dual-targeted nanomedicines to enhance tumor delivery efficacy is a complex challenge, largely due to the barrier posed by blood vessels during systemic delivery. Effective transport across endothelial cells is, therefore, a critical topic of study. Herein, we present a synthetic biology-based approach to engineer dual-targeted ferritin nanocages (Dt-FTn) for understanding receptor-mediated transport across tumor endothelial cells. By leveraging a genetically engineered logic-gated strategy, we coassembled various Dt-FTn in E. coli with tunable ratios of RGD-targeting and intrinsic TfR1-targeting ligands. These Dt-FTn constructs were employed to investigate the interaction between receptor-mediated vascular permeability and dual-targeted nanomedicines in low-permeability tumors. Through machine learning-based single vessel analysis, we uncovered the crucial role of dual-receptor expression profiles in determining the vascular transport of dual-targeted nanomedicines in tumors with low permeability. Using a patient-derived colon cancer model, we demonstrated a proof-of-concept that the optimal proportions of dual ligands in these nanomedicines can be customized based on tumor cell receptor expression profiles. This study provides valuable insights and guiding principles for the rational design of dual-targeted nanomedicines for tumor-targeted delivery.
提高低渗透肿瘤血管通透性的双靶向纳米药物的合理设计
设计双靶点纳米药物以提高肿瘤递送效率是一项复杂的挑战,主要是由于血管在全身递送过程中形成的屏障。因此,通过内皮细胞的有效运输是一个重要的研究课题。在此,我们提出了一种基于合成生物学的方法来设计双靶向铁蛋白纳米笼(Dt-FTn),以了解受体介导的肿瘤内皮细胞转运。通过利用基因工程逻辑门控策略,我们在大肠杆菌中共同组装了各种Dt-FTn,并可调节rgd靶向配体和内在tfr1靶向配体的比例。这些Dt-FTn结构被用来研究受体介导的血管通透性和双靶向纳米药物在低通透性肿瘤中的相互作用。通过基于机器学习的单血管分析,我们揭示了双受体表达谱在确定双靶向纳米药物在低通透性肿瘤中的血管运输中的关键作用。使用患者衍生的结肠癌模型,我们证明了概念证明,这些纳米药物中的双配体的最佳比例可以根据肿瘤细胞受体表达谱进行定制。本研究为肿瘤靶向递送的双靶向纳米药物的合理设计提供了有价值的见解和指导原则。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


