Vaccine-induced T cell responses control Orthoflavivirus challenge infection without neutralizing antibodies in humans
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
T cells have been identified as correlates of protection in viral infections. However, the level of vaccine-induced T cells needed and the extent to which they alone can control acute viral infection in humans remain uncertain. Here we conducted a double-blind, randomized controlled trial involving vaccination and challenge in 33 adult human volunteers, using the live–attenuated yellow fever (YF17D) and chimeric Japanese encephalitis–YF17D (JE/YF17D) vaccines. Both Orthoflavivirus vaccines share T cell epitopes but have different neutralizing antibody epitopes. The primary objective was to assess the extent to which vaccine-induced T cell responses, independent of neutralizing antibodies, were able to reduce post-challenge viral RNAaemia levels. Secondary objectives included an assessment of surrogate measures of viral control, including post-challenge antibody titres and symptomatic outcomes. YF17D vaccinees had reduced levels of JE/YF17D challenge viraemia, compared with those without previous YF17D vaccination (mean log10(area under the curve genome copies per ml): 2.23 versus 3.22; P = 0.039). Concomitantly, YF17D vaccinees had lower post-JE/YF17D challenge antibody titres that reduced JE virus plaque number by 50%, or PRNT50 (mean log10(PRNT50 titre): 1.87 versus 2.5; P < 0.0001) and symptomatic rates (6% (n = 1/16) versus 53% (n = 9/17), P = 0.007). There were no unexpected safety events. Importantly, after challenge infection, several vaccinees had undetectable viraemia and no seroconversion, even in the absence of neutralizing antibodies. Indeed, high vaccine-induced T cell responses, specifically against the capsid protein, were associated with a level of viral control conventionally interpreted as sterilizing immunity. Our findings reveal the importance of T cells in controlling acute viral infection and suggests a potential correlate of protection against orthoflaviviral infections. ClinicalTrials.gov registration: NCT05568953 . The authors demonstrate the effectiveness of T cells in controlling acute viral infections, without neutralizing antibodies, by conducting an Orthoflavivirus vaccination and challenge study in humans.

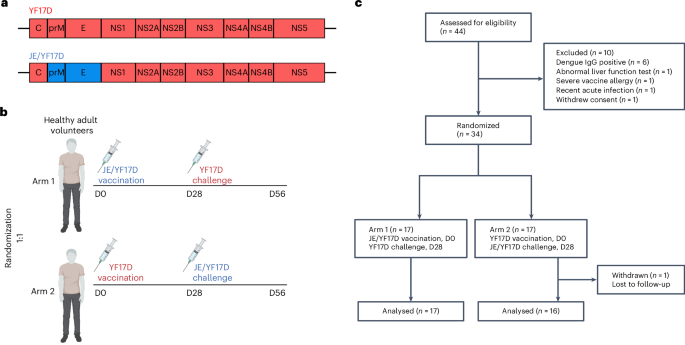
疫苗诱导的T细胞反应在没有中和抗体的情况下控制人类正黄病毒攻击感染
T细胞已被确定为保护病毒感染的相关因素。然而,疫苗诱导的T细胞所需的水平以及它们单独控制人类急性病毒感染的程度仍然不确定。在这里,我们进行了一项双盲,随机对照试验,涉及接种和攻击33名成年志愿者,使用减毒黄热病(YF17D)和嵌合日本脑炎- YF17D(乙脑/YF17D)疫苗。两种正黄病毒疫苗共享T细胞表位,但具有不同的中和抗体表位。主要目的是评估疫苗诱导的T细胞反应在多大程度上独立于中和抗体,能够降低攻击后病毒rnaemia水平。次要目的包括评估病毒控制的替代措施,包括攻毒后抗体滴度和症状结局。与以前未接种过YF17D疫苗的人相比,接种过YF17D疫苗的人乙脑/YF17D挑战病毒血症水平降低(平均log10(曲线下基因组拷贝数每ml的面积):2.23比3.22;p = 0.039)。同时,YF17D疫苗接种者的乙脑/YF17D攻击后抗体滴度较低,使乙脑病毒斑块数量或PRNT50降低50%(平均log10(PRNT50滴度):1.87比2.5;P & lt; 0.0001)和症状率(6%(1/16)和53% (9/17),P = 0.007)。没有意外的安全事件。重要的是,在攻击感染后,一些疫苗接种者无法检测到病毒血症,即使在没有中和抗体的情况下也没有血清转化。事实上,疫苗诱导的高T细胞反应,特别是针对衣壳蛋白的反应,与病毒控制水平有关,通常被解释为无菌免疫。我们的研究结果揭示了T细胞在控制急性病毒感染中的重要性,并提示了对正黄病毒感染的保护的潜在关联。ClinicalTrials.gov注册:NCT05568953。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


