In Silico-Guided Discovery of Polysaccharide Derivatives as Adjuvants in Nanoparticle Vaccines for Cancer Immunotherapy
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
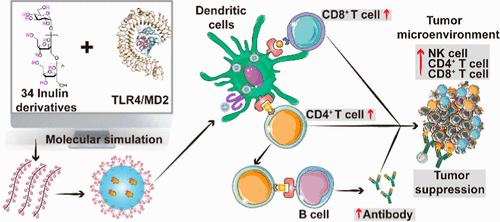
Cancer vaccines utilizing nanoparticle (NP) structures that integrate antigens and adjuvants to enhance delivery and stimulate immune responses are emerging as a promising avenue in cancer immunotherapy. However, the development of cancer vaccines has been significantly hindered by the low immunogenicity of tumor antigens. To address this challenge, substantial efforts have been made in developing innovative adjuvants to elicit effective immune responses. In this study, we develop a NP cancer vaccine assisted by a polysaccharide derivative adjuvant, designed through a computational strategy, to evoke effective antigen-specific antitumor immunity. Using TLR4 as the putative receptor, we conducted a comprehensive evaluation of a prescreening library consisting of 34 inulin derivatives through docking and molecular dynamics simulation. Consequently, a new derivative, benzoylated inulin (InBz), is selected as the most promising TLR4 agonist. The adjuvant effect of InBz is evaluated by fabricating InBz NPs encapsulating the model antigen ovalbumin (OVA). In vitro, InBz-OVA NPs effectively activate the TLR4 signaling pathways and facilitate dendritic cell maturation, thereby enhancing the antigen delivery and presentation. In vivo, InBz-OVA NPs outperform a commercial aluminum-based adjuvant, elicit robust antibody titers, induce antigen-specific cytotoxic T lymphocytes, and achieve significant tumor suppression in murine models. Besides, the adjuvant effects of other representative derivatives, namely, acetylated and chloroacetylated inulin, with moderate and low potential from the library, are also chemically synthesized and experimentally evaluated and found to be in agreement with computational predictions, confirming the credibility of the strategy. This study provides an effective platform for the pursuit of efficient polysaccharide-based vaccine adjuvants.
在硅引导下发现多糖衍生物作为癌症免疫治疗纳米颗粒疫苗的佐剂
利用纳米颗粒(NP)结构整合抗原和佐剂来增强递送和刺激免疫反应的癌症疫苗正在成为癌症免疫治疗中有前途的途径。然而,肿瘤抗原的低免疫原性严重阻碍了癌症疫苗的开发。为了应对这一挑战,在开发创新佐剂以引发有效免疫反应方面已经做出了大量努力。在这项研究中,我们开发了一种由多糖衍生物佐剂辅助的NP癌症疫苗,通过计算策略设计,以唤起有效的抗原特异性抗肿瘤免疫。以TLR4为推定受体,通过对接和分子动力学模拟,对包含34个菊粉衍生物的预筛选文库进行了综合评价。因此,一种新的衍生物苯甲酰化菊粉(InBz)被选为最有希望的TLR4激动剂。通过制备包封模型抗原卵清蛋白(OVA)的InBz NPs来评价InBz的佐剂作用。在体外实验中,InBz-OVA NPs有效激活TLR4信号通路,促进树突状细胞成熟,从而增强抗原的传递和提呈。在体内,InBz-OVA NPs优于商业铝基佐剂,引发强大的抗体滴度,诱导抗原特异性细胞毒性T淋巴细胞,并在小鼠模型中实现显着的肿瘤抑制。此外,还对该文库中具有中等和低电位的其他代表性衍生物乙酰化和氯乙酰化菊粉的辅助作用进行了化学合成和实验评价,结果与计算预测一致,证实了该策略的可信性。本研究为寻求高效的基于多糖的疫苗佐剂提供了一个有效的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
文献相关原料
公司名称
产品信息
阿拉丁
Inulin from dahlia tubers
阿拉丁
Fluorescein isothiocyanate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


