Maternal Exposure to Polystyrene Nanoplastics Disrupts Spermatogenesis in Mouse Offspring by Inducing Prdm14 Overexpression in Undifferentiated Spermatogonia
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Undifferentiated spermatogonia (Undiff-SPG) plays a critical role in maintaining continual spermatogenesis. However, the toxic effects and molecular mechanisms of maternal exposure to nanoplastics on offspring Undiff-SPG remain elusive. Here, we utilized a multiomics combined cytomorphological approach to explore the reproductive toxicity and mechanisms of polystyrene nanoplastics (PS-NPs) on offspring Undiff-SPG in mice after maternal exposure. The results indicated that PS-NPs decreased testosterone levels and reduced sperm concentration and quality in offspring male mice through maternal exposure. Moreover, PS-NPs could enter offspring Undiff-SPG, increase ROS levels, and decrease the viability of Undiff-SPG. According to the transcriptomics and proteomics analyses, PS-NPs caused offspring male mice Undiff-SPG inflammation by increasing the expression of Tnfsf18/Nlrp6. Mechanistically, we found that inflammation induced overexpression of the transcription factor Prdm14 in Undiff-SPG, which suppressed the expression of Ccdc33 and Tcirg1. Additionally, PS-NPs disrupted offspring spermatogenesis by inhibiting the Osbp2/Zcwpw1/Dhps expression. Furthermore, PS-NPs reduced the Undiff-SPG autophagic flux by reducing the expression of Igbp1/Gabarapl2. In conclusion, maternal exposure to PS-NPs caused inflammation in offspring Undiff-SPG, which resulted in Prdm14 overexpression that could disrupt spermatogenesis and normal autophagy.
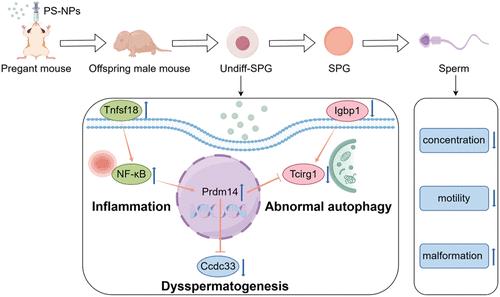
母体暴露于聚苯乙烯纳米塑料通过诱导未分化精原细胞中Prdm14的过表达来破坏小鼠后代的精子发生
未分化精原细胞(undiffi - spg)在维持精子持续发生中起着至关重要的作用。然而,母体接触纳米塑料对子代Undiff-SPG的毒性效应和分子机制尚不清楚。本研究采用多组学结合细胞形态学方法,探讨了聚苯乙烯纳米塑料(PS-NPs)对小鼠后代Undiff-SPG的生殖毒性及其机制。结果表明,PS-NPs通过母体接触降低了后代雄性小鼠的睾丸激素水平,降低了精子浓度和质量。PS-NPs可以进入后代undiffi - spg,增加ROS水平,降低undiffi - spg的存活率。转录组学和蛋白质组学分析表明,PS-NPs通过增加Tnfsf18/Nlrp6的表达引起子代雄性小鼠Undiff-SPG炎症。在机制上,我们发现炎症诱导Undiff-SPG中转录因子Prdm14的过表达,从而抑制Ccdc33和Tcirg1的表达。此外,PS-NPs通过抑制Osbp2/Zcwpw1/Dhps的表达来破坏子代精子发生。此外,PS-NPs通过降低Igbp1/Gabarapl2的表达来降低Undiff-SPG的自噬通量。综上所述,母体暴露于PS-NPs会引起后代undiffs - spg的炎症反应,导致Prdm14过表达,从而破坏精子发生和正常的自噬。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


