Manipulating the Adjacent Microenvironment of Atomically Dispersed FeN4 Sites via Cross-Link-Induced 3D Carbon Nest for Efficient Oxygen Reduction
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electronic perturbation induced by the microenvironment regulation adjacent to the FeN4 sites anchored on metal–N–C materials will accelerate its oxygen reduction reaction (ORR) kinetics. Herein, we report a fine-tuning in the charge configuration of FeN4 sites through a defect-rich N/S-doped carbon nest derived from the chemically cross-linked pyrrole/thiophene copolymer (CCPPT) with a sp3-hybridized cross-linker. Compared with the pyrrole/thiophene copolymer (PPT) without the cross-linker, CCPPT with a knitted three-dimensional (3D) network delivers higher defect density and ∼2-fold sulfur retention after pyrolysis. The structural characterizations combined with theoretical calculations suggest that adjacent vacancy defects (Cvd) and FeN4/S2 moiety together induce the charge redistribution of the FeN4 sites on the resultant CC-Fe1/NSC from CCPPT, reducing the adsorption strength of the oxygen-containing intermediates and the energy barrier of ORR. As expected, CC-Fe1/NSC shows an impressive half-wave potential of ∼0.91 V vs reversible hydrogen electrode (RHE), surpassing both the PPT-derived Fe1/NSC (0.88 V) and the commercial Pt/C (0.86 V). This work provides a distinctive path to manipulate the adjacent microenvironment of the single-atom catalysts toward ORR or even beyond.
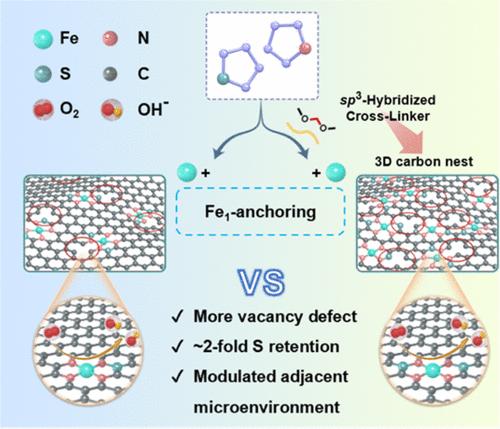
通过交联诱导的三维碳巢操纵原子分散的FeN4位点邻近微环境以实现高效的氧还原
金属- n - c材料上固定的FeN4位点附近的微环境调控引起的电子扰动将加速其氧还原反应(ORR)动力学。本文中,我们报道了一种由化学交联吡咯/噻吩共聚物(CCPPT)和sp3杂化交联剂衍生的富含缺陷的N/ s掺杂碳巢对FeN4位点的电荷配置进行微调。与没有交联剂的吡咯/噻吩共聚物(PPT)相比,具有编织三维(3D)网络的CCPPT在热解后具有更高的缺陷密度和约2倍的硫保留率。结构表征结合理论计算表明,相邻空位缺陷(Cvd)和FeN4/S2片段共同诱导CCPPT生成的CC-Fe1/NSC上FeN4位点的电荷重新分布,降低了含氧中间体的吸附强度和ORR的能垒。正如预期的那样,CC-Fe1/NSC对可逆氢电极(RHE)的半波电位为0.91 V,超过了Pt衍生的Fe1/NSC (0.88 V)和商用Pt/C (0.86 V)。这项工作提供了一种独特的途径来操纵单原子催化剂的邻近微环境,使其达到ORR甚至更高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


