Flexible Site-Specific Labeling-Mediated Self-Assembly Sensor Based on Quantum Dots and LUMinescent AntiBody Sensor for Duplexed Detection of Antibodies
IF 8.2
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
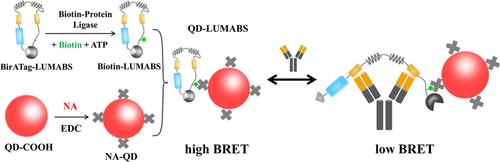
Over recent years, the LUMinescent AntiBody Sensor (LUMABS) system, utilizing bioluminescence resonance energy transfer (BRET), has emerged as a highly effective method for antibody detection. This system incorporates NanoLuc (Nluc) as the donor and fluorescent protein (FP) as the acceptor. However, the limited Stokes shift of FP poses a challenge, as it leads to significant spectral cross-talk between the excitation and emission spectra. This issue complicates the implementation of multiplexed detection. To address this challenge, we present an innovative enhancement to the LUMABS sensor with quantum dots (QDs) as the acceptor instead of FP. The use of QDs offers several advantages over those of traditional FP-based sensors. The biotin–avidin system facilitates the flexible interchangeability of QDs, allowing for a more convenient multicolor sensor construct. The new QD-LUMABS system overcomes the limitations of spectral cross-talk and provides better spectral separation. This breakthrough enables the successful implementation of multiplexed detection for multiple targets simultaneously. Results demonstrated that the wavelength-tunable QD-LUMABS sensors achieved picomolar-level detection limits for antibodies and that this sensor-construction strategy was generally applicable among various epitopes and their antibodies. Furthermore, this sensor displayed excellent duplexing capabilities. These features underscore its potential for future clinical disease diagnosis applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


