Dissolved beryllium (< 1 kDa) mobilized as a major element in groundwater in legacy mine waste
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Research regarding the geochemistry of beryllium (Be) in terrestrial environments is hindered by its high toxicity to humans and the low concentrations normally occurring in the environment. Although Be is considered an immobile element, extremely high dissolved concentrations have been detected in groundwater in the legacy Tailings Storage Facility (TSF) of Smaltjärnen, Sweden. Therefore, a detailed study was conducted to determine physiochemical parameters affecting the speciation of Be in the groundwater. Groundwater was sampled from 2016–2024 and filtered through 0.2 μm filters, whereas truly dissolved fraction (<1 kDa) samples were collected with dialysis membrane tubes in situ at groundwater wells. Secondary minerals on the tailings shore were studied by mineralogical methods and sequential extraction to trace the pathway whereby Be entered the downstream surface water. In part of the tailings, dissolved Be was detected in very high concentrations (average: 4.8 mg/L) in suboxic groundwater with pH from 6.0 to 6.4. Dialysis sampling in 2024 showed that more than 90% occurred as truly dissolved Be (<1 kDa). A significant correlation between Be and S was found, suggesting that sulfate complexes kept Be mobile in these pH conditions. Dissolved Be increased with decreased pH, and there is risk that the concentrations will increase further since sulfide oxidation with subsequent decrease in pH will continue for 100 of years in the TSF. In another part of the TSF, the pH was > 6.4 and dissolved Be was below the detection limit, possibly due to formation of Al(OH)3 (>0.2 μm) together with F and Zn. Secondary minerals on the shore of the tailings functioned as a temporary chemical barrier, scavenging Be primarily by secondary gypsum when present and otherwise by Fe hydr(oxides).
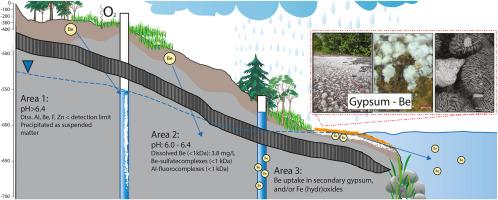
矿渣中溶解铍(< 1 kDa)作为主要元素在地下水中运移
铍(Be)在陆地环境中的地球化学研究因其对人类的高毒性和环境中通常存在的低浓度而受到阻碍。虽然Be被认为是一种不可移动的元素,但在瑞典Smaltjärnen的传统尾矿储存设施(TSF)的地下水中检测到极高的溶解浓度。因此,对影响地下水中Be形态的理化参数进行了详细的研究。2016-2024年抽取地下水,通过0.2 μm过滤器过滤,而真正溶解部分(<1 kDa)的样品则在地下水井中使用透析膜管原位采集。采用矿物学方法和序贯萃取法对尾矿岸上的次生矿物进行了研究,追踪了Be进入下游地表水的途径。部分尾矿在pH为6.0 ~ 6.4的亚氧地下水中检测到高浓度溶解Be(平均4.8 mg/L)。2024年透析取样显示90%以上为真正溶解Be (<1 kDa)。Be和S之间存在显著的相关性,表明硫酸盐配合物在这些pH条件下保持Be的流动性。溶解Be随着pH值的降低而增加,并且存在浓度进一步增加的风险,因为硫化物氧化随着pH值的降低将在TSF中持续100年。在TSF的另一部分,pH为>;6.4和溶解Be低于检出限,可能是由于Al(OH)3 (>0.2 μm)与F和Zn一起形成。尾矿岸边的二次矿物起着临时化学屏障的作用,当存在时,主要通过二次石膏清除Be,否则则通过铁水合物(氧化物)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


