An Atomistic Analysis of the Carpet Growth of KCl Across Step Edges on the Ag(111) Surface
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
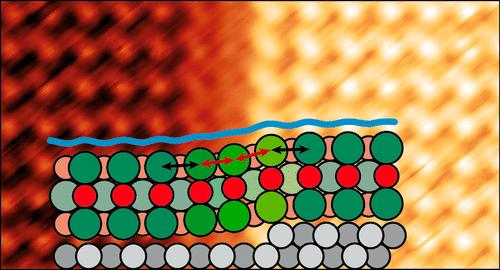
The carpet growth of alkali halide (AH) layers across step edges of substrates enables the growth of seamless and continuous large domains. Yet, information about how the AH layer adapts continuously to the height difference between the terraces on the two sides of a step is only described by continuum models, which do not give details of the ionic displacements. Here, we present a first study of thin epitaxial KCl(100) layers grown on the Ag(111) surface by scanning tunneling microscopy that provides atomistic details for the first time. Measurements were performed at room temperature. Using a Cl–-decorated tip, we resolved the ionic arrangement and hence the KCl lattice distortion in the carpet growth region, in some cases even by imaging both types of ions. Our findings demonstrate the ability of the KCl lattice to distort locally over a short distance of four KCl unit cells as a result of the attractive interaction between the ions and the Ag atoms at and close to the steps. For Ag step edges covered by the KCl carpet, we observe a tendency to straighten along the ⟨110⟩ direction of the KCl layer. In addition, the carpet growth induces the formation of Ag microterraces, i.e., the splitting of higher Ag steps into multiple Ag steps of monatomic height during the KCl deposition at elevated temperatures. These microterraces have a minimum width determined by an energetically preferred fitting to the KCl lattice and allow for the carpet growth, while growth across higher Ag steps is not observed.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


