Facile Aqueous Route to Large-Scale Superhydrophilic TiO2-Incorporated Graphitic Carbon Nitride-Coated Ni(OH)2 and Ni2P Nano-Architecture Arrays as Efficient Electrocatalysts for Enhanced Hydrogen Production
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
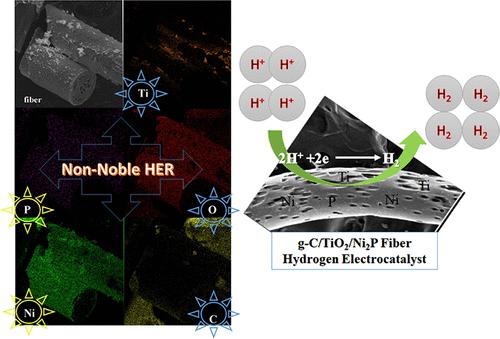
Water splitting by an electrochemical method to generate hydrogen gas is an economic and green approach to resolve the looming energy and environmental crisis. Designing a composite electrocatalyst having integrated multichannel charge separation, robust stability, and low-cost facile scalability could be considered to address the issue of electrochemical hydrogen evolution. Herein, we report a superhydrophilic, noble-metal-free bimetallic nanostructure TiO2/Ni2P coated on graphitic polyacrylonitrile carbon fibers (g-C/TiO2/Ni2P) using a facile hydrothermal method followed by phosphorylation. In an aqueous-based route, PAN is dissolved in water in the presence of ZnCl2, followed by wet-spinning to prepare scalable PAN/ZnCl2 fibers. The nitrogen-contained porous graphitic carbon fibers are prepared via the pyrolysis of PAN/ZnCl2 fibers; now ZnCl2 acts as a volatile porogen to form porous matrix structures. Finally, the as-prepared graphitic carbon fibers are electrochemically activated by incorporating TiO2/Ni2P active sites. The materials formed in this work show excellent electrocatalytic activity for the hydrogen evolution reaction. The as-synthesized g-C/TiO2/Ni2P catalyst shows a low overpotential, its electrocatalytic activity is improved, and its efficiency is better than that of the commercial Pt/C catalyst. At a current density of −10 mA/cm2, the g-C/TiO2/Ni2P catalyst shows an overpotential of 55 mV, while the commercial Pt/C catalyst shows an overpotential of 77 mV. Our work provides a facile aqueous scalable route with no need for noble metals that can be considered as a potential alternative for the commercial Pt/C catalyst.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


