Spontaneous Bubble Growth Inside High-Saturation-Vapor-Pressure and High-Air-Solubility Liquids and Emulsion Droplets
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
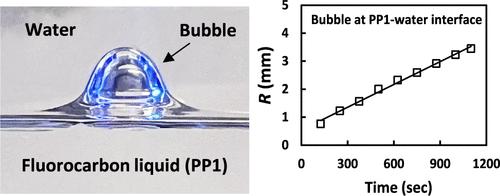
Spontaneous bubble growths in liquids are usually triggered by rapid changes in pressure or temperature that can lead to liquid gas supersaturation. Here, we report alternative scenarios of the spontaneous growths of bubbles inside a high-saturation-vapor-pressure and high-air-solubility perfluorocarbon liquid (PP1) that were observed under ambient quiescent conditions. First, we investigate spontaneous bubble growth inside the single PP1 phase, which was left to evaporate freely. The bubble growth is explained by the difference in the PP1 vapor pressure inside the bubble and that above the freely evaporating PP1 interface. Next, we study the bubble growth inside the liquid PP1 covered with a layer of a second air-saturated immiscible liquid: low-air-solubility water or higher-air-solubility ethanol. In both cases, the bubble growth rates were accelerated, indicating mass transfer of air from the water or ethanol phases to the PP1 phase. The bubble growth rates significantly increase for bubbles trapped at the PP1–water or PP1–ethanol interfaces due to faster air diffusion through the thin PP1 liquid films separating the bubbles from the upper phases. Finally, we consider the case of bubbles inside millimeter-sized PP1 emulsion droplets in water or ethanol. The bubble growth inside the droplet leads to an increase in the PP1 droplet’s effective buoyancy and to the detachment of the droplets from the substrate. The observed bubble growth rate in the case of emulsion droplets was much faster for PP1 droplets in ethanol than for PP1 droplets in water (minutes vs hours). The underlying physical mechanism of the increase of bubble volumes is the spontaneous mass transfer of both air and PP1 vapor to the bubbles produced by a colloidal diffusion pump effect.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


