Reexamining the Enhanced Solubility of Sodium Laurate/Sodium Oleate Eutectic Mixtures
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mixtures of multiple surfactants that have superior performance to the individual components are highly sought-after commercially. Mixtures with a reduced Krafft point (TK) are particularly useful as they enable applications at lower temperatures. Such an example is the soap maker’s eutectic: the mixture of sodium laurate (NaL) and sodium oleate (NaOl). A true eutectic implies that the two surfactants do not mix in the solid state but mix readily in the micellar solution above TK, leading to a sharp TK depression at a specific composition. However, the NaL/NaOl mixture shows a broad TK depression of >15 °C at a NaOl weight fraction (wO) of about 0.5. Our tie-line analysis shows that pure NaL and NaOl do not coexist in the solid phase on either side of the TK minimum. X-ray analysis of the isolated solids with varying wO reveals that a unique intermediate compound (I.C.) forms in the solid state with a NaL-to-NaOl mole ratio of about 4/3. Below the TK minimum, NaL and the I.C. coexist in the solids for wO < 0.5, whereas the I.C. and NaOl coexist in the solids for wO > 0.5. Each pair of solids exhibits eutectic or monotectic solubility behavior, and the congruent I.C. melting point is so close to that of the eutectic point(s) that a broad TK minimum ensues. Thermal analysis and modeling via the freezing-point depression approach support the above interpretation. The fact that surfactants with other headgroups but the same blend of chain lengths do not exhibit similar depressed TK is a topic for further study.
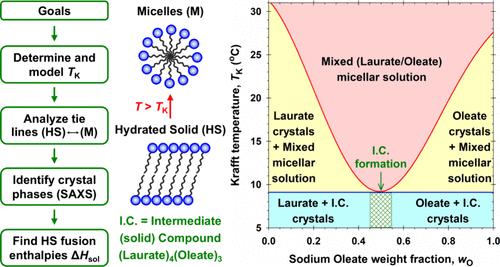
月桂酸钠/油酸钠共晶混合物增强溶解度的再研究
多种表面活性剂的混合物具有优于单个组分的性能,在商业上非常受欢迎。降低卡夫点(TK)的混合物特别有用,因为它们可以在较低的温度下应用。这样的一个例子是肥皂制造商的共晶:月桂酸钠(NaL)和油酸钠(NaOl)的混合物。真正的共晶意味着这两种表面活性剂在固体状态下不混合,而是在TK上方的胶束溶液中容易混合,导致特定组成的TK急剧下降。然而,NaL/NaOl混合物在NaOl质量分数(wO)约为0.5时,TK明显降低了15°C。我们的联线分析表明,纯NaL和NaOl在TK最小值两侧的固相中不共存。x射线分析表明,在不同wO的分离固体中,形成了一种独特的中间化合物(ic),其nal与naol的摩尔比约为4/3。在TK最小值以下,al和ic在固体中同时存在;0.5,而ic和NaOl在固体中共存;0.5. 每一对固体都表现出共晶或单晶的溶解度行为,并且一致的熔点与共晶点非常接近,从而产生了一个宽的TK最小值。通过凝固点下降方法进行的热分析和建模支持上述解释。具有其他头基但链长相同的表面活性剂没有表现出类似的抑制TK,这是一个有待进一步研究的课题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


