Probing the Redox Reactivity of Alkyl Bound Astatine: A Study on the Formation and Cleavage of a Stable At–C Bond
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
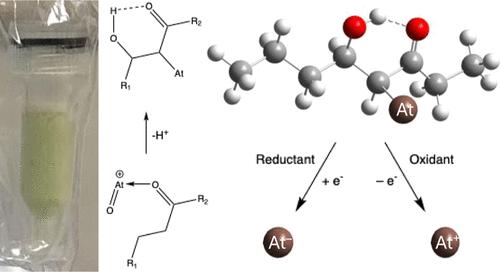
The formation of a stable alkyl At–C bond occurs during the shipment of 211At on a 3-octanone-impregnated column and the reactivity of 211At stripped from columns has been studied. The 211At could not be recovered from the 3-octanone organic phase using nitric acid or sodium hydroxide, even up to 10 and 15.7 M, respectively. Several reducing and oxidizing agents, including hydrazine, hydroxylamine, ascorbic acid, ceric ammonium nitrate, potassium permanganate, sodium hypochlorite, and calcium hypochlorite were used to promote the recovery of 211At. The most effective reducing agent was hydroxylamine, where ∼70% of the 211At was recovered, while among oxidizing agents ceric ammonium nitrate, potassium permanganate, and sodium hypochlorite all showed near quantitative recovery of 211At. These results indicate an At–C bond is being formed during the shipment of the column and a redox reaction is required for bond cleavage to occur. DFT calculations have been used to propose several products of an AtO+-3-octanone reaction, with 4-astato-5-hydroxy-octa-3-one being the most probable.
探讨烷基结合砹的氧化还原反应活性:稳定At-C键的形成和裂解研究
在3-辛酮浸渍柱上运送2111at时,形成了稳定的烷基At-C键,并研究了从柱上提取的2111at的反应性。使用硝酸和氢氧化钠,即使在10 M和15.7 M的条件下,也不能从3-辛酮有机相中回收211At。用肼、羟胺、抗坏血酸、硝酸铈铵、高锰酸钾、次氯酸钠、次氯酸钙等还原剂和氧化剂促进了211At的回收。最有效的还原剂是羟胺,其中约70%的211At被回收,而在氧化剂中,硝酸铈铵、高锰酸钾和次氯酸钠都显示出接近2111at的定量回收。这些结果表明,在色谱柱的运输过程中形成了At-C键,并且需要氧化还原反应才能发生键裂解。DFT计算已经提出了AtO+-3-辛酮反应的几种产物,其中4-astato-5-羟基-辛酮-3- 1是最有可能的产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


