Tyrosine additives with rich-polar functional groups provide multi-protections for ultra-stable zinc metal anodes
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
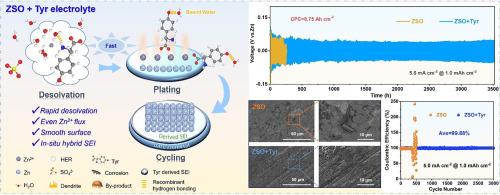
The widespread commercialization of aqueous zinc-ion batteries (AZIBs) is severely limited by dendrite growth and rampant parasitic reactions. While various additives have been introduced to improve the stability of zinc anodes, a single polar functional group additive cannot provide comprehensive protection for zinc anodes. Here, a low-cost, high-functionality Tyrosine (Tyr) organic small molecule is utilized as an electrolyte additive to achieve ultra-stable zinc metal anodes. The findings indicated that the electronegative carboxyl group tended to participate in the solvation sheath of Zn2+. The zinc-philic amino group and hydrophobic benzene ring synergistically construct a hydrophobic electric double layer on the surface of the zinc anode. The hydrophilic nature of the hydroxyl group enables it to capture free water molecules and reconstruct the hydrogen bonding network of the electrolyte. More importantly, the strong adsorption of Tyr molecule is beneficial to induce the formation of an in-situ organic-inorganic hybrid solid electrolyte interface layer, thereby further enhancing protection for the zinc anode. Profiting from the synergistic effect of the polyfunctional group in the Tyr additive, the Zn||Zn cell exhibits ultra-long cycle stability over 3800 h (∼ 20 times vs. ZnSO4) at 1.0 mA cm‒2, 1 mAh cm‒2 and an ultra-high cumulative plated capacity of 8.75 Ah cm‒2 at 5.0 mA cm−2. Furthermore, the Zn||Cu cell delivers a significantly improved reversibility with an average Coulomb efficiency of 99.88% after 3000 cycles. This finely regulated electrolyte, leveraging the synergistic effects of multiple functional groups, heralds a promising trajectory for the advancement of enduring Zn metal batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


