Ternary Metal (W–Ni–Sr) Oxide@Polypyrrole Nanotubes: A New Frontier in the Electrochemical Detection of Promethazine Hydrochloride (PMHC)
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
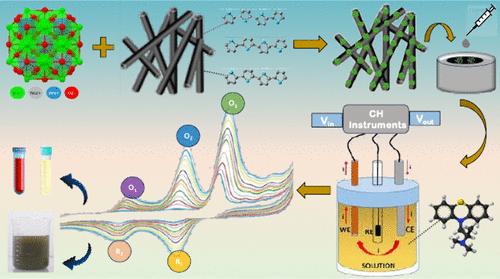
Promethazine hydrochloride (PMHC) is a vital drug that is used as an anticholinergic, antipsychotic, antihistaminic, analgesic, sedative, and neuroleptic. However, the overdosage of PMHC also causes reproductive variations, cardiac changes, hypotension, and endocrinal variations. Hence, the detection of PMHC is crucial. Therefore, in this work an electrochemical method for the detection of PMHC is reported. The fabrication for the modified electrode is built with tungsten (W), nickel (N), and strontium (S) ternary oxide (tWNSO). To the best of our knowledge, this tWNSO ternary oxide preparation is reported for the first time in the literature. The prepared ternary oxide is deposited on the polypyrrole nanotubes, and this nanocomposite that is formed is confirmed by various physical characterizations like XRD, SEM, TEM, UV–vis spectroscopy, FTIR spectroscopy, and also DFT studies for PMHC. Thus, the nanocomposite obtained is used as a working electrode for the detection of PMHC. The fabricated tWNSO/PPyNTs/GCE has an effective surface area of 0.0436 cm2. Also, no fouling was observed. The limit of detection of the analyte PMHC is 3.66 nM, the limit of quantification is 11.10 nM, and the sensitivity of the fabricated electrode in identifying the analyte is found to be 20.10 μA μM–1 cm–2. Thus, the modified working electrode effectively detects the analyte PMHC while demonstrating excellent stability and reproducibility.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


