Multi-effect synergistic induction of unsaturated MnOx on sandy sediment for enhanced manganese adsorption and byproduct resource recovery in solar evaporation
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The efficient removal of Mn(II) from wastewater is crucial for safeguarding water quality, yet existing adsorbents face significant challenges, including high costs, poor resistance to ionic interference, and scalability limitations. This study addresses these challenges by utilizing abundant natural sandy sediment (SS) as a substrate to load unsaturated MnOx via in-situ oxidation, creating a novel adsorbent (MOSS). MOSS exhibits a remarkable Mn(II) adsorption capacity of 1.35 mg·g−1, representing a 6–7-fold increase compared to SS. The unsaturated nature of MnOx in MOSS enables effective co-separation of transition metals, further enhancing its utility. It is observed that redox reactions, metal complex formation, and ions exchange processes may play a significant role in further enhancing its adsorption capacity and selectivity. In dynamic filtration tests, MOSS effectively maintains Mn(II) removal below 0.1 mg·L−1 after continuous effluent and retains over 50 % separation efficiency after three regeneration cycles. And the byproducts of Mn(II) adsorption were successfully repurposed as a photo-thermal material for solar evaporator, achieving an evaporation rate of 1.97 kg·h−1·m−2 and a conversion efficiency of 98.64 %. This study presents a cost-effective, scalable, and sustainable method for Mn(II) removal, while offering novel insights into the high-value utilization of MOSS byproducts for environmental remediation and resource recovery.
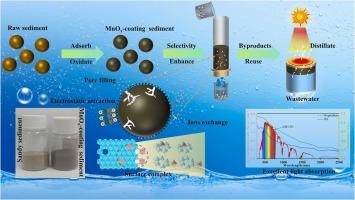
非饱和MnOx多效协同诱导砂质沉积物增强对锰的吸附和太阳蒸发副产物资源的回收
有效去除废水中的锰(II)对于保护水质至关重要,但现有的吸附剂面临着重大挑战,包括成本高、抗离子干扰能力差以及可扩展性限制。本研究通过利用丰富的天然砂质沉积物(SS)作为基质,通过原位氧化加载不饱和MnOx,创造了一种新型吸附剂(MOSS),解决了这些挑战。MOSS对Mn(II)的吸附能力为1.35 mg·g-1,比SS提高了6-7倍。MOSS中MnOx的不饱和特性使其能够有效地共分离过渡金属,进一步提高了其实用性。观察到氧化还原反应、金属配合物形成和离子交换过程可能对进一步提高其吸附能力和选择性起重要作用。在动态过滤试验中,连续出水后,MOSS有效地将Mn(II)去除率保持在0.1 mg·L-1以下,三次再生循环后,分离效率保持在50%以上。吸附Mn(II)的副产物成功地用作太阳能蒸发器的光热材料,蒸发器的蒸发速率为1.97 kg·h-1·m-2,转化效率为98.64%。本研究提出了一种具有成本效益、可扩展且可持续的Mn(II)去除方法,同时为MOSS副产品在环境修复和资源回收中的高价值利用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


