Controlled Growth of Silver Nanoparticles by Metal–Support Interaction for Enhanced Tandem Catalytic Oxidation of HCHO at Low Temperature
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
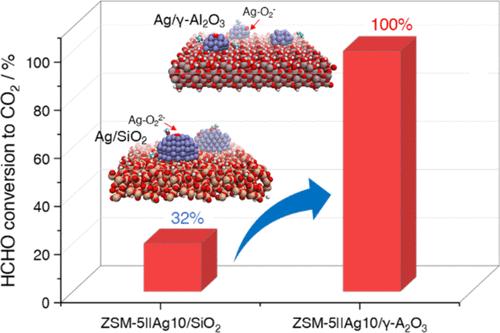
Bifunctional zeolite-Ag catalysts with a tandem process offer a versatile pathway for efficient HCHO removal at low temperature. The overall performance of tandem systems is highly sensitive to the tunable nature of the Ag component. Herein, we report a tandem ZSM-5||Ag/γ-Al2O3 catalyst that exhibits superior low-temperature activity compared to the ZSM-5||Ag/SiO2 catalyst, achieving 100% conversion at 55 °C versus 32% for the latter. This dramatic improvement is attributed to the morphological variations induced by differences in the mobility and dispersion of supported Ag nanoparticles. This process involves a complex interplay between the properties of metal nanoparticles (NPs) and the supports. Combining experiments and advanced ab initio molecular dynamics simulations (AIMD), the control of Ag NPs growth behaviors by regulating metal–support interaction (MSI) strength and surface diffusion on different supports is revealed. Strong MSI and high diffusion barrier on γ-Al2O3 triggers the slow Ostwald ripening (OR), whereas weak MSI and low diffusion barrier on SiO2 stimulates the facile particle migration and coalescence (PMC), leading to rapid activity decay. The observed activity difference is strongly related to the surface activation of MF intermediates and the O2 molecule, which was controlled by Ag NPs size. These findings demonstrate the feasibility and efficacy of controlling MSI strength for the design of stable and high-performance supported metal catalysts.
金属-载体相互作用调控银纳米颗粒生长对HCHO串联催化氧化的影响
双功能沸石-银催化剂的串联工艺提供了一种高效的低温脱除HCHO的通用途径。串联系统的整体性能对Ag元件的可调谐特性高度敏感。在此,我们报道了一种串联ZSM-5||Ag/γ-Al2O3催化剂,与ZSM-5||Ag/SiO2催化剂相比,它表现出更好的低温活性,在55℃下转化率达到100%,而后者为32%。这种显著的改善是由于负载银纳米颗粒的迁移率和分散性的差异引起的形态变化。这一过程涉及到金属纳米颗粒(NPs)和载体之间复杂的相互作用。结合实验和先进的从头算分子动力学模拟(AIMD),揭示了通过调节金属-载体相互作用(MSI)强度和在不同载体上的表面扩散来控制Ag NPs的生长行为。γ-Al2O3表面强的MSI和高的扩散势垒触发了缓慢的Ostwald成熟(OR),而SiO2表面弱的MSI和低的扩散势垒则刺激了粒子的易迁移和聚并(PMC),导致活性快速衰减。观察到的活性差异与MF中间体和O2分子的表面活化密切相关,而O2分子的表面活化受Ag NPs大小的控制。这些发现证明了控制MSI强度设计稳定、高性能负载金属催化剂的可行性和有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


