Lowering the Kinetic Barrier via the Synergistic Catalysis of N-CNTs Supported RhP Subnanoclusters and Confined Co Nanoparticles for Olefins Hydroformylation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
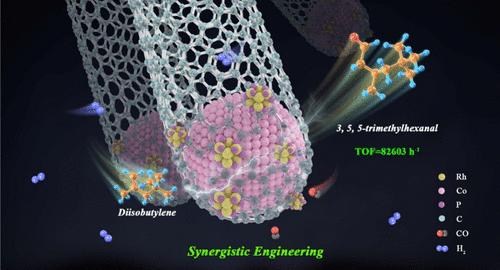
Developing synergistic sites for a multistep elementary reaction is important, but challenging. Herein, the coexistence of Co nanoparticles confined inside the carbon nanotubes and RhP subnanoclusters loaded on the outside of carbon nanotube (Co@N-CNTs|RhP) is synthesized by a defect-assisted impregnation strategy. Notably, Co nanoparticles and RhP subnanoclusters have a synergistic engineering distance of 0.66 nm. The turnover frequency over Co@N-CNTs|RhP catalyst could reach 82603 h–1 during the diisobutylene hydroformylation, 15 times higher than that of the commercial Rh homogeneous catalysts. More importantly, the obtained Co@N-CNTs|RhP achieved 25 catalytic cycles. Kinetic experiments show that the synergistic engineering of confined Co nanoparticles and RhP subnanoclusters is the key to promote the hydroformylation of diisobutylene, which reduces the activation energy to 64.6 kJ/mol. Further in situ DRIFT spectra and theoretical calculations reveal the confined Co nanoparticles modified by RhP subnanoclusters are conducive to the adsorption of H2, CO, and diisobutylene, while the RhP subnanoclusters are responsible for the formation of aldehydes. This work provides profound insight for the construction of efficient multisite heterogeneous catalyst for long-chain olefin conversion reactions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


