Structural basis for the conformational protection of nitrogenase from O2
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
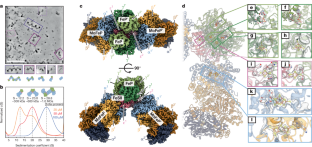
The low reduction potentials required for the reduction of dinitrogen (N2) render metal-based nitrogen-fixation catalysts vulnerable to irreversible damage by dioxygen (O2)1–3. Such O2 sensitivity represents a major conundrum for the enzyme nitrogenase, as a large fraction of nitrogen-fixing organisms are either obligate aerobes or closely associated with O2-respiring organisms to support the high energy demand of catalytic N2 reduction4. To counter O2 damage to nitrogenase, diazotrophs use O2 scavengers, exploit compartmentalization or maintain high respiration rates to minimize intracellular O2 concentrations4–8. A last line of damage control is provided by the ‘conformational protection’ mechanism9, in which a [2Fe:2S] ferredoxin-family protein termed FeSII (ref. 10) is activated under O2 stress to form an O2-resistant complex with the nitrogenase component proteins11,12. Despite previous insights, the molecular basis for the conformational O2 protection of nitrogenase and the mechanism of FeSII activation are not understood. Here we report the structural characterization of the Azotobacter vinelandii FeSII–nitrogenase complex by cryo-electron microscopy. Our studies reveal a core complex consisting of two molybdenum–iron proteins (MoFePs), two iron proteins (FePs) and one FeSII homodimer, which polymerize into extended filaments. In this three-protein complex, FeSII mediates an extensive network of interactions with MoFeP and FeP to position their iron–sulphur clusters in catalytically inactive but O2-protected states. The architecture of the FeSII–nitrogenase complex is confirmed by solution studies, which further indicate that the activation of FeSII involves an oxidation-induced conformational change. Cryo-electron microscopy of Azotobacter vinelandii FeSII–nitrogenase reveals a core complex of molybdenum–iron proteins (MoFePs), iron proteins (FePs) and FeSII, in which FeSII mediates interactions with MoFeP and FeP to position their FeS clusters in catalytically inactive but O2-protected states.

氮酶对O2的构象保护的结构基础
二氮(N2)还原所需的低还原电位使得金属基固氮催化剂容易受到二氧(O2)1,2,3的不可逆损伤。这种对O2的敏感性是氮酶的一个主要难题,因为大部分固氮生物要么是专性需氧生物,要么与呼吸O2的生物密切相关,以支持催化N2还原的高能量需求4。为了对抗氧气对氮酶的损害,重氮营养体使用氧气清除剂,利用区隔化或保持高呼吸速率来减少细胞内的氧气浓度。最后一种损害控制是由“构象保护”机制提供的,在该机制中,一种称为FeSII的[2Fe:2S]铁氧化还原蛋白家族蛋白(参考文献10)在O2胁迫下被激活,与氮酶成分蛋白形成抗O2复合物11,12。尽管有前人的见解,但氮酶构象O2保护的分子基础和FeSII激活的机制尚不清楚。在这里,我们报告了用低温电子显微镜对固氮菌(Azotobacter vinelandii) fesii -氮酶复合物的结构表征。我们的研究揭示了一个由两个钼铁蛋白(MoFePs)、两个铁蛋白(FePs)和一个FeSII同型二聚体组成的核心复合物,它们聚合成延伸的细丝。在这个三蛋白复合物中,FeSII介导了与MoFeP和FeP相互作用的广泛网络,将它们的铁硫簇定位在催化无活性但o2保护的状态。溶液研究证实了FeSII -氮酶复合物的结构,这进一步表明FeSII的激活涉及氧化诱导的构象变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


