RNA control of reverse transcription in a diversity-generating retroelement
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
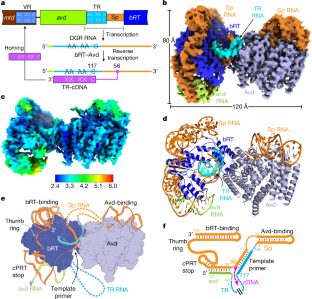
Diversity-generating retroelements (DGRs) create massive protein sequence variation (up to 1030)1 in ecologically diverse microorganisms. A recent survey identified around 31,000 DGRs from more than 1,500 bacterial and archaeal genera, constituting more than 90 environment types2. DGRs are especially enriched in the human gut microbiome2,3 and nano-sized microorganisms that seem to comprise most microbial life and maintain DGRs despite reduced genomes4,5. DGRs are also implicated in the emergence of multicellularity6,7. Variation occurs during reverse transcription of a protein-encoding RNA template coupled to misincorporation at adenosines. In the prototypical Bordetella bacteriophage DGR, the template must be surrounded by upstream and downstream RNA segments for complementary DNA synthesis to be carried out by a complex of the DGR reverse transcriptase bRT and associated protein Avd. The function of the surrounding RNA was unknown. Here we show through cryogenic electron microscopy that this RNA envelops bRT and lies over the barrel-shaped Avd, forming an intimate ribonucleoprotein. An abundance of essential interactions in the ribonucleoprotein precisely position an RNA homoduplex in the bRT active site for initiation of reverse transcription. Our results explain how the surrounding RNA primes complementary DNA synthesis, promotes processivity, terminates polymerization and strictly limits mutagenesis to specific proteins through mechanisms that are probably conserved in DGRs belonging to distant taxa. This study presents cryogenic electron microscopy structural analysis of a diversity-generating retroelement (DGR) reverse transcription system from Bordetella bacteriophage, with results indicating that RNA surrounding the template controls reverse transcription through mechanisms that are conserved across distant taxa.

在一个多样性产生的逆转录元件中逆转录的RNA控制
多样性生成逆转录因子(DGRs)在生态多样化的微生物中产生大量的蛋白质序列变异(高达1030)1。最近的一项调查发现,来自1500多种细菌和古细菌属的大约31000个dgr,构成了90多种环境类型2。dgr尤其富集于人类肠道微生物组2,3和纳米级微生物中,这些微生物似乎构成了大多数微生物生命,尽管基因组减少了,但仍能维持dgr 4,5。dgr也与多细胞性的出现有关6,7。变异发生在蛋白质编码RNA模板的逆转录过程中,与腺苷的错误结合偶联。在典型的博德氏菌噬菌体DGR中,模板必须被上游和下游RNA片段包围,以便DGR逆转录酶bRT和相关蛋白Avd的复合体进行互补DNA合成。周围RNA的功能是未知的。在这里,我们通过低温电子显微镜显示,这种RNA包裹在bRT上,并位于桶状的Avd上,形成了一个亲密的核糖核蛋白。核糖核蛋白中大量的基本相互作用精确地定位了bRT活性位点上的RNA同源双工,以启动逆转录。我们的研究结果解释了周围RNA如何启动互补DNA合成,促进加工,终止聚合并严格限制特定蛋白质的突变,其机制可能在属于遥远分类群的dgr中保守。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


