Single Precursor-Derived Sub-1 nm MoCo Bimetallic Particles Decorated on Phosphide–Carbon Nitride Framework for Sustainable Hydrogen Generation
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The strategic design and fabrication of efficient electrocatalysts are pivotal for advancing the field of electrochemical water splitting (EWS). To enhance EWS performance, integrating non-noble transition metal catalysts through a cooperative double metal incorporation strategy is important and offers a compelling alternative to conventional precious metal-based materials. This study introduces a novel, straightforward, single-step process for fabricating a bimetallic MoCo catalyst integrated within a three-dimensional (3D) nanoporous network of N, P-doped carbon nitride derived from a self-contained precursor. The subsequent carbonization at 550 °C yields a highly effective bimetallic phosphide carbon nitride electrocatalyst, denoted as MoCoPCN, tailored explicitly for EWS. The MoCoPCN electrocatalyst demonstrates exceptional electrocatalytic performance, with a low onset potential of 1.43 V and an overpotential value of 202 mV at a current density of 10 mA/cm2 for the oxygen evolution reaction (OER) and 49.5 mV for the hydrogen evolution reaction (HER), respectively. Moreover, the catalyst exhibits a high electrochemically active surface area of 2720 cm–2, a small Tafel slope of 47.5 mV dec–1 for HER and 45.7 mV dec–1 for the OER, and a low charge transfer resistance of 0.09 Ω for the HER and 0.805 Ω for the OER. The optimal catalyst was tested for overall water splitting performance in a 1 M KOH electrolyte, demonstrating excellent efficiency with a low cell voltage of 1.49 V required to achieve a current density of 10 mA/cm2. These outstanding characteristics, combined with the synergistic effects arising from the interaction between MoCo and P-g-C3N4 (PCN), underscore the potential of a bimetallic phosphide carbon nitride material as a highly promising electrocatalyst for efficient water splitting.
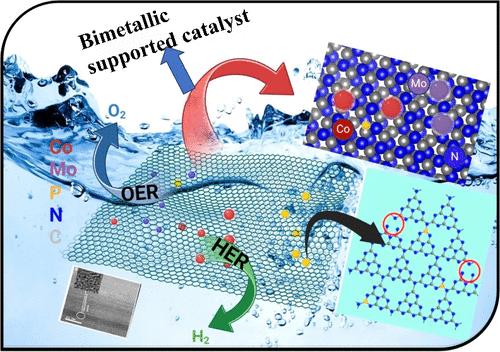
单前驱体衍生的亚- 1nm MoCo双金属粒子在磷化碳-氮化碳框架上修饰,用于可持续制氢
高效电催化剂的战略性设计和制造是推进电化学水分解领域发展的关键。为了提高EWS的性能,通过双金属结合策略整合非贵金属过渡金属催化剂非常重要,并为传统的贵金属基材料提供了令人信服的替代方案。本研究介绍了一种新颖、直接、单步的工艺,用于制造双金属MoCo催化剂,该催化剂集成在三维(3D)纳米孔网络中,该网络由自含前驱体衍生的N, p掺杂氮化碳。随后在550℃下碳化,得到了一种高效的双金属磷化碳氮电催化剂,标记为MoCoPCN,专门为EWS量身定制。MoCoPCN电催化剂表现出优异的电催化性能,在电流密度为10 mA/cm2时,析氧反应(OER)的起始电位为1.43 V,过电位为202 mV,析氢反应(HER)的过电位为49.5 mV。此外,该催化剂具有2720 cm-2的高电化学活性表面积,HER的Tafel斜率为47.5 mV dec1, OER的Tafel斜率为45.7 mV dec1, HER和OER的电荷转移电阻分别为0.09 Ω和0.805 Ω。在1 M KOH电解液中测试了最佳催化剂的整体水分解性能,结果表明,在1.49 V的低电池电压下,实现了10 mA/cm2的电流密度。这些突出的特性,加上MoCo和P-g-C3N4 (PCN)之间相互作用产生的协同效应,强调了双金属磷化碳氮材料作为高效水分解电催化剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


