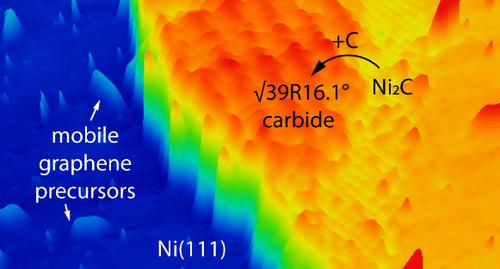
Initial Carbonation of Ni(111) Surfaces
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding the carbon formation on Ni surfaces is critical for the controlled Ni-based nanofabrication and heterogeneous catalysis. Due to the high solubility of carbon in nickel and the complicated migrations of carbon in the near-surface area, achieving a fundamental understanding of the initial carbonation of a Ni surface at an atomic level is experimentally challenging. Herein, the initial formation of surface carbon adsorbates on Ni(111) from the Boudouard reaction (2CO ↔ CO2 + C) is studied by scanning tunneling microscopy (STM) in combination with density functional theory (DFT) calculations. The initial carbon formation is site-selective: carbon adsorption at step edges is isolated and strongly bonded, acting as the precursor of carbide formation; the adsorption on terrace sites is weaker and mobile, acting as the initial graphene clusters on Ni(111). The difference in kinetics of C adsorption on the Ni(111) may play a role in determining the future growth of carbide or graphene. Upon further carbon adsorption, new evidence is presented to resolve the debate over the atomic structure of the well-recognized (√39 × √39) R16.1° carbide structure. Our results based on combined STM measurements and DFT calculations are further extended to other surfaces, such as Ni(110) and Ni(211), and a wide range of temperatures and pressures. This provides valuable insights into controlling the chemical processes related to carbon–nickel interactions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


