Molecular Origin of Nanoscale Anion Ordering of LiTFSI Electrolytes Revealed through SAXS/WAXS and Molecular Dynamics Simulations
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
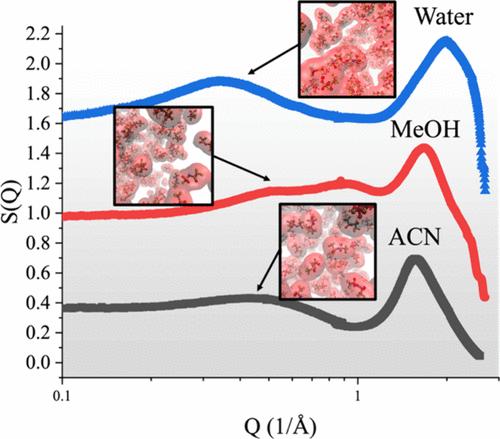
Recent developments in “water-in-salt” electrolytes have precipitated a renewed effort to study imide-based electrolytes. While previous small-/wide-angle X-ray scattering (SAXS/WAXS) studies have attributed the emergence of a low-Q peak in the SAXS profile of aqueous LiTFSI electrolytes to nanometer-scale anion clustering, a molecular-level understanding of the root of these clusters remains unclear. In this study, we combined molecular dynamics simulations and SAXS/WAXS to study the solvation structures of LiTFSI in acetonitrile, methanol, and water. We concluded that hydrogen bonding in water and MeOH stabilizes anion clusters, while nonpolar methyl groups on methanol and acetonitrile interrupt the nanoscale ordering of TFSI anions. This causes LiTFSI in water and MeOH electrolytes to exhibit two low-Q SAXS profile peaks while LiTFSI in acetonitrile exhibits only a single peak below Q = 1 Å–1. These findings shed light on the underlying molecular origins of nanoscale anion clusters, which may help in the design of the next generation of electrolyte chemistries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


