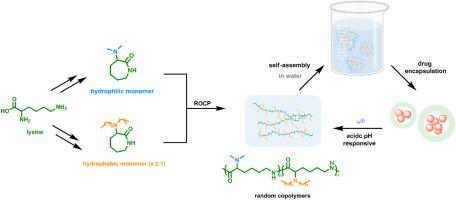
Synthesis of lysine-based amphiphilic random copolymers capable of self-assembly in water
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
A series of copolymers featuring polyamide-6 skeleton were synthesized through the ring-opening copolymerization of seven membered cyclic lactam monomers derived from lysine with varying degrees of hydrophilicity. Dimethyl protected cyclic lysine (DMCL) serves as a certainly hydrophilic comonomer, while the hydrophobic comonomer bears longer substitutive alkyl groups on the side nitrogen atom, such as dibutyl protected cyclic lysine (DBCL). Both calculations and experimental results indicate the resulted copolymers possess random structures. Nevertheless, amphiphilic copolymers can be achieved by reasonably adjusting the length of the alkyl chain in the hydrophobic monomer and flexibly varying the ratio of hydrophilic and hydrophobic units. Notably, poly(DMCL-co-DBCL)73, which has a hydrophilic to hydrophobic ratio of 7 to 3, is capable of self-assembling into well-defined micelles in water and showed excellent potential for drug delivery applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


